Article Text
Abstract
Introduction Compensatory mouth breathing, caused by nasopharyngeal obstructive diseases, is the main cause of hyperdivergent mandibular retrognathia in children. Such deformities require effective growth guidance before pubertal growth peaks. The traditional mandibular advancement device, twin block (TB), can guide the forward development of the mandible. However, the side effect of increasing the vertical dimension of the lower facial third, worsens the facial profile of children with divergent growth trends. To solve this problem, a modified TB (LLTB) appliance was designed to control the vertical dimension by intruding incisors and inhibiting the elongation of posterior teeth during the advancement of the mandible, which could avoid the side effects of traditional appliances and effectively guide the growth of the mandible in a normal direction.
Methods and analysis The study was designed as a single-centre, single-blind, randomised, parallel controlled trial. We aim to enrol 60 children aged 9–14 years with hyperdivergent skeletal class II malocclusion, using a 1:1 allocation ratio. The participants were will be randomly assigned to receive either the TB or LLTB treatment. The primary outcome will be a change in the angle of the mandibular plane relative to the anterior cranial base. The secondary outcomes will include changes in the sagittal maxillomandibular relation, occlusal plane, facial height, morphology of the mandible and upper airway width. Safety endpoints will also be evaluated.
Ethics and dissemination Ethical approval was obtained from the ethics committee of Shanghai Stomatological Hospital. Both participants and their guardians will be fully informed of the study and sign an informed consent form before participating in the trial. The results will be publicly available in peer-reviewed scientific journals.
Trial registration number ChiCTR2000035882.
- ORAL MEDICINE
- Paediatric oral & maxillofacial surgery
- SLEEP MEDICINE
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Selection bias will be minimised by designing a randomised controlled trial to compare the efficacy of modified twin block (TB) with conventional TB in children with hyperdivergent mandibular retrognathia.
This study will help orthodontists choose mandibular advancement devices especially for those children with long-face growth patterns.
A key limitation is the inability to blind the researchers involved in treatment.
Introduction
Convex deformity is a common facial deformity in children. The treatment of children with long-face growth patterns also remains to be a challenging clinical problem. In recent years, the number of children with nasopharyngeal airway obstructive diseases has been increasing, and the resulting compensatory mouth breathing is the main cause of dental and maxillofacial deformities in these children.1
We investigated the prevalence of deciduous dentition malocclusion among children aged between 3 and 5 years in Shanghai and analysed the correlation between malocclusion and oral habits, dietary structure and upper respiratory diseases.2 Researchers have found that chronic rhinitis and adenotonsillar hypertrophy are highly correlated with mouth breathing,3 accompanied by a higher prevalence of hyperdivergent malocclusion.4
Children are in an important stage of dental and maxillofacial development and respiratory pattern formation.5 Long-term airway obstruction and mouth breathing can cause narrowing of the maxillary dental arch, increased anterior face height, lip incompetence, and backward and downward mandibular rotation, thus resulting in mandibular retrognathia.6 7 Mandibular retrognathia is an important risk factor for sleep apnoea, which can affect children’s behavioural psychology and social ability.8 Such deformities have serious physical and psychological effects on children and their parents.9 If effective growth modification is not carried out before the adolescent growth spurt, only orthognathic surgery can achieve satisfactory results in adulthood.10
Hyperdivergent convex deformity in children, caused by long-term mouth breathing,11 is one of the difficulties in orthodontic treatment.12 13 The current clinical methods for mandibular growth guidance mainly include twin block (TB), activator and Herbst, whose main function is to protrude the mandible and stimulate its growth.14–16 However, the above traditional mandibular advancement devices (MADs) have obvious limitations in the treatment of children with long-face growth patterns.17 For children with deep overbite before treatment, the forward movement of the mandible is accompanied by an open bite of the posterior teeth, which may lead to continued elongation of the posterior teeth, an increase in the inferior height and deterioration of the profile.18 Therefore, traditional MADs are not applicable to patients with hyperdivergent mandibular retrognathia.19
To overcome these difficulties, the modified TB (LLTB, figure 1) appliance has been designed and patented (Yun Lu, Yuehua Liu, Qiang Li, Tingchao Lan, Min Zhao, Huanhuan Li. Double-bite blocks appliance, 12 November 2019, China, ZL201821348135.7). In the process of guiding the forward development of the mandible, the LLTB appliance can simultaneously intrude the upper and lower anterior teeth, flatten the inclined occlusal plane (OP), avoid elongation of the posterior teeth during the occlusal adjustment process, and effectively guide the growth direction of the mandible. LLTB has been used in clinical cases of hyperdivergent mandibular retrognathia, and the effect of this modified appliance needs to be systematically analysed. This study aims to analyse and compare the clinical effects of TB and LLTB in children with hyperdivergent mandibular retrognathia for the purpose of providing valuable information to guide the clinical treatment of such cases. Compared with the previous studies that emphasised sagittal orientation,18 20 21 this study focuses on the vertical control in the mandibular advancement of hyperdivergent mandibular retrognathia.
Modified twin block (LLTB) appliance. LLTB, modified twin block.
Methods and analysis
Study design
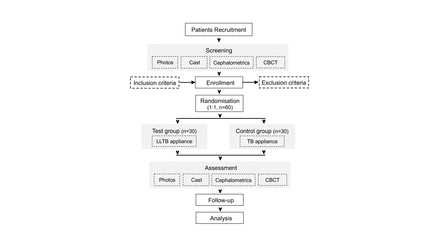
This study is designed as a single-centre, single-blind, randomised parallel controlled trial, aiming to evaluate the efficacy of a modified LLTB and compare its effect with the traditional TB in children with hyperdivergent mandibular retrognathia. The study is registered at http://www.chictr.org.cn/index.aspx, which can be accessed online. The trial will be performed in the Department of Orthodontics at Shanghai Stomatological Hospital. We will recruit 60 patients who meet the inclusion criteria and randomly assign them to one of two treatment groups, TB or LLTB, in a 1:1 ratio. Treatment in both groups will last for 12 months, with follow-up visits every 2 months. Participants will be assessed at the following time points: the baseline (before treatment), the end of the treatment (after 12 months of treatment) and follow-up (6 months after treatment is completed). A brief flow chart of the study is provided in figure 2 and the trial schedule is presented in online supplemental table 1. The design adheres to the Consolidated Standards of Reporting Trials statement recommendations. If there will be significant modifications to the eligibility criteria, outcomes and analyses arising from the implementation of this study, relevant investigators, trial registries, journals and regulators will be notified.
Supplemental material
Flow chart of the study. CBCT, cone beam CT; TB, twin block.
Patient and public involvement
There was no patient or public involvement in the design, recruitment or conduct of this study. After the treatment, researchers will communicate with the participants and their guardians about the efficacy and follow-up arrangements.
Study patient
Participants will be recruited from the Orthodontic Department of Shanghai Stomatological Hospital via a specific referral pathway. Patients and their guardians will be informed about hyperdivergent mandibular retrognathia and this study through outpatient consultations and recruitment posters in the hospital. Interested individuals will be able to meet with research assistants who will explain the study in detail, perform an initial screening and obtain informed consent. Subsequently, the orthodontists will conduct clinical examinations and cephalometric analysis for the participants to obtain parameters related to the inclusion criteria, which ultimately determine whether the participants will be included in this study. A total of 60 eligible patients will be recruited for the study after screening. The following eligibility criteria were developed to ensure the precision of the results:
Inclusion criteria
Aged 9–14, no gender limit.
Protrusion deformity, poor chin morphology, mandibular retrusion, ANB>5° and normal maxillary development.
Children with a high mandibular plane angle (SN-MP, the inclination of the mandibular plane [MP] to the sella–nasion [SN] line, which is an indicator of the vertical proportion of the face), 35°<SN-MP<45°. Children treated with traditional TB are prone to elongation of the posterior teeth, causing the backward and downward rotation of the mandible. The increase in facial height will aggravate the original long face and cause ethical issues; therefore, the SN-MP is controlled within 45°. Participants included in this study are children showing a tendency of a long-face growth pattern, but have not yet developed into severe long faces. Even if the facial height increases after treatment with TB, it is still acceptable.
Narrow upper airway.
Participants have good compliance and are able to wear appliances as required and rechecked regularly.
Exclusion criteria
Children who have passed pubertal peak according to the modified cervical spine analysis method (Cvs4 stage).
Children who have symptoms of temporomandibular joint disorders.
Children with loose deciduous molar.
Patients with systemic disease.
Recruitment and randomisation process
Participants will be recruited through outpatient clinics and hospital-based advertisements. If the participants and their guardians are interested in participating in the trial, they will sign an informed consent form after fully understanding the study. A pretreatment screening visit with a clinical research assistant will be conducted in the outpatient clinic before enrollment. Once considered eligible for enrolment, the enrolled participants will be randomly assigned to one of the two treatment groups, either TB or LLTB, in a 1:1 ratio with block randomisation method. The independent statistician will use SAS V.9.4 software to generate a random allocation table with flexible block size according to the total sample size. Random assignment and concealment will be performed by interactive web response system system (http://redcap.fudan.edu.cn).
Description of the interventions
Orthodontic treatments in both groups will be performed by experienced orthodontists following a consistent protocol. In the test group, an LLTB appliance combined with brackets and archwires will be used to intrude the upper anterior teeth, flatten the inclined OP, and guide the mandible to rotate counterclockwise with the adjustment of the blocks. Participants in the control group will wear the TB appliance every day without adjustment of the anterior teeth. The treatments will last for 12 months, with follow-up every 2 months. Dental casts, digital oral and facial photographs, lateral radiographs, and cone beam CT images will be obtained before and after treatment. Third-party interpreters will perform cephalometrics, and the dental-skeletal-soft tissue parameters will be comprehensively analysed in the sagittal, transverse and vertical dimensions.
Treatment group
Participants in the trial group will be treated with LLTB for mandibular advancement. The LLTB appliance in the trial group provides three-dimensional guidance and control to the growing children from vertical, sagittal and transverse directions by the auxiliary intrusion archwire, bite blocks and rapid maxillary expansion respectively, which is different from the traditional TB appliance focusing on sagittal advancement. The fixed LLTB appliance is designed with two double buccal tubes in the posterior region, which are connected to the anterior brackets by an archwire. We use 0.012-inch or 0.014-inch nickel-titanium wire as primary archwire to align the anterior teeth, and 0.016-inch stainless steel wire as intrusion archwire to intrude the upper anteriors and level the OP in the anterior region. Along with the intrusion of the anterior teeth, bite blocks will be grinded successively to lower the height and guide the mandible to rotate forward and upward to improve the chin profile.
Control group
The participants in the control group will be treated with TB for mandibular advancement. The TB appliance consists of removable maxillary and mandibular plates with ramps that guide the mandible forward. The maxillary plate incorporates an expansion screw to increase posterior arch width. Due to the limitations of the removable appliance, TB cannot achieve a large amount of maxillary expansion. In addition, the removable TB is unable to achieve anterior teeth adjustment and, thus, will not guide the mandible to rotate counterclockwise during the treatment. Only the Adams clasps will be adjusted for good retention at each follow-up visit.
Outcome measures
The measurement outcomes in this study are mainly derived from the comparison of cephalometric measurements on lateral radiographs before and after treatment, with photographs and dental casts as auxiliary references.
Primary outcome
The primary outcome of this study is the degree of change in the SN-MP. SN-MP is the angle of intersection of the mandibular plane and the anterior cranial base plane, which indicates the steepness of the mandibular plane and the height of the lower facial third and thus can reflect the vertical growth pattern. The larger the SN-MP, the greater the amount of vertical growth and the corresponding increase in treatment difficulty. The SN-MP is a commonly used parameter for evaluating the downward and backward rotation of the mandible and is also a valid indicator for assessing the efficacy of vertical control.
Secondary outcomes
The secondary outcomes include the following aspects:
The ANB angle: In cephalometric analysis, points A and B represent the sagittal position of the maxilla and mandible, respectively. The ANB angle represents the anterior–posterior relationship between the maxilla and mandible.
The angle of the OP (OP-SN): The angle OP-SN is used to establish the relationship of the OP to the cranial base. OP-SN represents the inclination of the OP, reflecting the relative relationship between the vertical dimension of the anterior and posterior teeth. The larger the angle, the steeper the OP and the more serious the tendency of the protrusion type.
Tooth height analysis: The vertical height of the teeth will be analysed by measuring the distance of the upper and lower anterior teeth and the posterior teeth relative to the reference plane using U1-PP, U6-PP, L1-MP and L6-MP. The analyses of the above parameters before and after treatment reflect the influence of different appliances on tooth heights.
Facial height analysis: Facial height will be analysed by two parameters, S-Go/N-Me and ANS-Me/N-Me, which represent the ratio of posterior face height to anterior face height and the percentage of lower face height to total face height, respectively. MADs have effects on posterior face height (S-Go) and lower face height (ANS-Me). In order to reflect the effect of face height changes on facial contours, the ratio of S-Go and ANS-Me to total face height (N-Me) will be evaluated.
Mandibular morphology: The changes in mandibular morphology will be analysed by measuring the mandibular length (Co-Gn), ramus height (Ar-Go), mandibular body length (Go-Me) and Gonial angle (Ar-Go'-Me). The mandibular morphology will be evaluated by the combination of linear and angular measures. Co-Gn represents the overall length of the mandible, while Ar-Go and Go-Me reflect the vertical and sagittal development of the mandible respectively. Ar-Go'-Me reflects the morphology of the chin, a parameter that has a critical influence on the lateral profile.
The facial convexity angle (Ns-Sn-Pos): The angle Ns-Sn-Pos will be measured to assess lateral protrusion of the soft tissue. This parameter is based on nassion of soft tissue (Ns), subnasal (Sn) and pogonion of sott tissue (Pos) and evaluates the sagittal change of soft tissue chin with respect to the lateral profile.
Upper airway analysis: MADs can adjust the position of the hyoid bone by stimulating mandibular growth, increasing glossopharyngeal and hypopharyngeal airway. TB-TPPW and V-LPW measurements will be used to assess the widths of the middle and lower segments of the upper airway.
Safety monitoring
The MADs used in both groups in this study have no obvious adverse effects on the participants if they are worn according to the doctor’s advice. Possible risks are mainly the adverse effects of poor oral hygiene during orthodontic treatment, such as enamel demineralisation, gingivitis and gingival hyperplasia. Mild enamel demineralisation and gingivitis can be alleviated by timely measures such as oral cleaning and hygiene promotion. Participants with severe enamel demineralisation and caries should stop wearing the appliance and continue with mandibular advancement after filling treatment. Patients suffering from severe gingivitis with gingival hyperplasia should be terminated from the study and undergo periodontal scaling. Gingival trimming will be performed to cure gingival hyperplasia in patients with no obvious relief after periodontal scaling for 1 month.
Sample size calculation
Based on the data from a pilot study, the primary outcome is the change of SN-MP at 12 months post-treatment relative to that before treatment, with a mean difference of 1.5° and an SD of 1.87°. Using a conventional α of 5% and β of 20%, 26 participants are required for each group. With a drop-out rate of 10%, the number of participants in each group is 30, and the final sample size for this study is 60 participants, randomised in a 1:1 ratio.
Statistical analysis
During the measurement process, statistical analysts will be blinded to the participants' personal information and group assignments. Statistical significance will be set at p<0.05, based on a two-sided test. Continuous variables will be described as mean±SD or median (P25–P75), depending on whether they conform to a normal distribution. All statistical analyses will be performed using the statistical software SPSS V.26.0.
All analyses will be based on iIntention-to-treat principle. The primary analysis strategy for the primary and secondary outcomes will be the mixed-effect model with baseline adjustment. Multiple imputation will be used as sensitivity analysis. Safety endpoints will be compared by χ2 test or Fisher’s exact test. Subgroup analysis stratified by age and gender is preplanned.
Ethics and dissemination
The study has been approved by the ethics committee of Shanghai Stomatological Hospital (approval no. (2021)023; (2022)005) and will comply with the Declaration of Helsinki. All amendments to the programme will be implemented with the approval of the ethics committee. All the participants and their guardians will be fully informed of the study and sign an informed consent form before joining the study. They will be informed that they can withdraw from the study at any time without explanation.
The results will be publicly available in peer-reviewed scientific journals and presented at academic conferences. Any public reporting of study results will not disclose personal information about participants.
Discussion
The influence of breathing patterns on maxillofacial growth and development has been confirmed by the previous studies. Compared with nasal breathing, mouth breathing causes children and adolescents to show vertical growth patterns with increased facial height and a retrognathic mandible with a high mandibular plane angle due to backward and downward rotation.22 23
Currently, it is difficult to treat children with hyperdivergent mandibular retrognathia. Orthodontists can guide and improve mandibular growth in the adolescent growth spurt by making good use of the growth potential.24 However, due to the backward rotation of the mandible and infra-anterior inclination of the OP, the effect of mandibular advancement in children with hyperdivergent mandibular retrognathia is not satisfactory.25 Increase in vertical dimension is the most obvious change in the children with vertical growth patterns. Traditional MADs such as activator or TB, not only stimulate mandibular growth in the sagittal direction but also cause an increase in the vertical direction, which makes the long-face pattern more severe.17 18 Studies have shown that children of long-face type are more susceptible to vertical growth stimuli.26 It has been reported that the high-pull headgear and chin cup can control the vertical growth of the mandible,27 and present cephalometric outcome stability of treatment even after a long-term follow-up.28 Lione confirmed that posterior bite-blocks could reduce the vertical dimension in children with a high mandibular angle after treatment with rapid maxillary expansion.29 However, the study of Cha et al revealed that patients with severe hyperdivergent mandibular retrognathia showed very similar vertical maxillofacial results regardless of whether or not they were treated with functional appliances, manifesting a clinical tendency for a higher mandibular plane and excessive eruption of teeth after treatment.30
Therefore, the treatment for children with long-face growth patterns should incorporate vertical control methods that may help prevent the continued increase in the mandibular plane and improve the facial profile in both sagittal and vertical dimensions. Most studies on vertical control have focused on orthognathic surgery and compensatory orthodontic treatment in adult patients with high mandibular plane angles.31 32 But few studies have investigated the improvement in mandibular advancement in children with hyperdivergent mandibular retrognathia. Zervas et al found that the cervical headgear showed more control over the vertical dimension and produced more favourable changes in mandibular position by normalising the OP in children with class II division 1 malocclusion.33 However, this study did not involve a significant sagittal discrepancy between the maxilla and the mandible. A review of the literature found that studies on modified functional appliances were limited to patients with normal growth patterns,34 35 and there was a lack of systematic high-quality studies on children of long-face growth patterns.
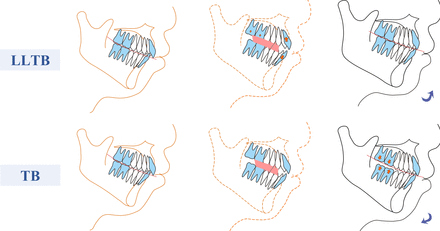
For children with hyperdivergent mandibular retrognathia, a modified LLTB appliance was designed to take vertical control measures to intrude incisors and inhibit the elongation of posterior teeth during mandibular advancement, which can avoid the side effects of the traditional appliance and effectively guide the growth of the mandible in a normal direction. The comparison of treatment mechanisms between LLTB and TB is shown in figure 3. The effect of LLTB on growth improvement and vertical control needs to be systematically analysed and evaluated in a large number of cases. In this study, a single-centre, randomised, single-blind, parallel controlled trial is conducted to verify the efficacy of the new appliance in improving dentomaxillofacial growth guidance in children with hyperdivergent retrusive mandibles. This study focuses on comparing the efficacy of modified and traditional appliances. The randomised controlled trial in this study is expected to clarify the potential benefits of the modified appliance over conventional appliances. The evaluation will include sagittal and vertical analysis of the teeth, jaws, facial soft tissues, and upper airway width measurement.
The comparison of treatment mechanisms between LLTB and TB. LLTB, modified twin block; TB, twin block.
Since the primary differences between the two groups are the appliance and procedure, it is not possible to blind the orthodontists who will perform the treatment. To minimise trial bias, we will blind the patients and data surveyors, and the data will be averaged from repeated measurements by a third-party reader.
Ethics statements
Patient consent for publication
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors YLiu was responsible for the trial design and protocol writing, YLu supervised the study protocol, and read and reviewed the manuscript. AL participated in the study design and helped draft the manuscript; WZ contributed in randomisation and group assignment design, statistical consultation and manuscript revision; WZ obtained ethical approval; SS participated in the study and coordination; and ZC is responsible for the table and schematic diagrams. All authors have read and approved the final manuscript.
Funding This study was supported by the Medical Innovation Research Project of the Science and Technology Commission of Shanghai Municipality (grant number 21Y11903600) and the Clinical Innovation Project of Shanghai Shenkang Hospital Development Center (grant number SHDC12021108).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.