Article Text
Abstract
Objective Evaluate the feasibility of a trial of perioperative hypotension and serious complications.
Design A patient and assessor-blinded randomised feasibility trial.
Setting We included patients in a tertiary university hospital.
Participants We enrolled 80 adults scheduled for major non-cardiac surgery.
Interventions In patients randomised to tight blood pressure control, intraoperative mean arterial pressure (MAP) was targeted to ≥85 mm Hg maintained with norepinephrine infusion, and restarting chronic antihypertensive medications was delayed until the third postoperative day. In the reference group, intraoperative blood pressure was managed per routine and antihypertensive medications were restarted immediately after surgery.
Primary and secondary outcome measures Our first co-primary outcome was the fraction of time when intraoperative MAP was >85 mm Hg, intraoperative area (time integral) of MAP >85 mm Hg and MAP <65 mm Hg. The second co-primary outcome was time until antihypertensive medications were restarted after surgery. Secondary outcomes were time-weighted average intraoperative MAP, cumulative minimum MAP for 10 min, average postoperative systolic blood pressure (SBP) and mean of the lowest three postoperative SBPs.
Results Forty patients in each group were analysed. The median for intraoperative area of MAP >85 mm Hg was 1303 (772–2419) mm Hg*min in routine blood pressure (BP) cases and 2425 (1926–3545) mm Hg*min in tight BP control. The area for intraoperative MAP <65 mm Hg was 7 (0–40) mm Hg*min with routine BP management, and 0 (0–0) mm Hg*min with tight BP control. The fraction of time with MAP >85 mm Hg was 0.52 (0.25) and 0.87 (0.15). Antihypertensive medications were restarted 2 (1–3) days later in tight BP control cases. However, postoperative SBPs were similar.
Conclusions Tight BP management markedly increased intraoperative MAP and reduced the amount of hypotension. In contrast, delaying chronic antihypertensive medications had little effect on postoperative SBP. The full trial appears feasible and remains necessary but should not include postoperative antihypertensive management.
Trial registration NCT04789733.
- Adult anaesthesia
- Hypertension
- Ischaemic heart disease
Data availability statement
Data are available upon reasonable request. The investigators and statistician have access to the whole data set. Data will be shared collaboratively with external parties with the approval of the Executive Committee and appropriate data-use agreements.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The protocol was practical, and intraoperative pressure management resulted in excellent separation.
However, we failed to manipulate postoperative blood pressure by changing when antihypertensive medications were restarted.
Feasibility in one site does not mean that it will prove practical at all trial sites.
Introduction
Mortality in the 30 days after surgery is more than 100 times higher than intraoperative mortality.1 Myocardial injury and associated vascular complications are among the leading causes of postoperative mortality.2 Intraoperative hypotension is associated with myocardial injury after non-cardiac surgery (MINS) and myocardial infarction (MI), with the apparent harm threshold being a mean arterial pressure (MAP) ≈65 mm Hg.3 4 Furthermore, postoperative hypotension is associated with MI even after adjustment for intraoperative hypotension.5
The harm threshold for perioperative acute kidney injury (AKI) also appears to be an MAP near 65 mm Hg.6 Perioperative hypotension is also associated with delirium and cognitive decline,7 8 although inconsistently.9 Furthermore, cumulative duration of MAP less than 50, 55, 60, 70 and 80 mm Hg appears associated with increased odds of 30-day mortality after non-cardiac surgery is reported in a retrospective cohort.4 Hypotension prevention may therefore be a modifiable factor that reduces postoperative cardiovascular and perfusion-related complications.
There is currently sparse evidence that the associations observed between hypotension and myocardial and renal injury are casual. A small randomised trial (n=292) reports that preventing intraoperative hypotension reduces the risk of major complications by 25%.10 In contrast, a 458-patient randomised trial demonstrated no improvement with tight intraoperative blood pressure control.11 Limited randomised data (n=199) also suggests that hypotension causes delirium.12
A robust trial remains necessary to characterise the potential benefits of reducing perioperative hypotension in high-risk patients. We therefore plan a multinational randomised trial to test the primary hypothesis that perioperative hypotension prevention in high-risk patients reduces a composite of perfusion-related complications in the 30 days after major non-cardiac surgery. In anticipation of the full trial, we conducted a pre-planned feasibility trial—reported here—designed to evaluate the feasibility, especially the ability to target blood pressure per protocol.
Methods and analysis: participants, intervention and outcomes
Study design
This single-centre trial was performed in China-Japan Union Hospital of Jilin University (Jilin, China). The trial was registered prior to patient enrolment at clinicaltrials.gov (named as The GUARDIAN Pilot Trial, Principal investigator: KL).
Inclusion criteria
Major inclusion criteria were age ≥45 years; non-cardiac surgery expected to last at least 2 hours; overnight hospitalisation; American Society of Anesthesiologists (ASA) physical status 2–4; chronically taking at least one anti-hypertensive medication and expected to have an arterial catheter before anaesthesia induction. Participants were also required to have at least one of the following risk factors: (1) history of peripheral arterial surgery; (2) history of coronary artery disease; (3) history of stroke or transient ischaemic attack; (4) serum creatinine >175 µmol/L (>2.0 mg/dL); (5) diabetes requiring medication; (6) current smoking or 15 pack-year history of smoking tobacco; (7) scheduled for major vascular surgery; (8) body mass index ≥35 kg/m2; (9) preoperative high-sensitivity troponin T >14 ng/L or troponin I equivalent or (10) B-type natriuretic protein >80 ng/L or N-terminal B-type natriuretic protein >100 ng/L.
Exclusion criteria
Patients were excluded when they were scheduled for carotid artery surgery, intracranial surgery, partial or complete nephrectomy, pheochromocytoma surgery or liver transplantation. Patients were similarly excluded if they had a condition that precluded routine or tight blood pressure management or had end-stage renal disease. And finally, we also excluded patients with dementia or impairments that might compromise cognitive assessments.
Randomisation and masking
Participants were randomly allocated using computer-generated assignments to tight or routine pressure management in a 1:1 ratio without stratification in a block size of four by an independent statistician (DSY) using SAS V.9.2 software (SAS Institute). Allocation was concealed within sealed opaque envelopes until shortly before anaesthesia induction.
Intervention
The original protocol is detailed in online supplemental text document. No changes were made before trial data were accessed. This manuscript adheres to the applicable CONSORT guidelines.
Supplemental material
Our feasibility trial was designed to inform a future pivotal trial by considering two co-primary feasibility hypotheses. First, there is suitable statistically significant and clinically meaningful separation of intraoperative and postoperative blood pressure across two blood pressure management strategies (intraoperative MAP maintained ≥85 mm Hg and postoperative tight pressure control vs routine care with some hypotension expected). And second, restarting routine antihypertensive medications per protocol is feasible (restart delayed until the third postoperative day vs immediate restart). We also consider the exploratory efficacy hypothesis that perfusion-related complications and delirium are reduced by tight perioperative blood pressure control.
In patients assigned to tight pressure management, ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) were not given on the morning of surgery. A norepinephrine peripherally intravenous infusion was adjusted to maintain intraoperative MAP ≥85 mm Hg. Either intermittent bolus 4–8 µg norepinephrine at 2 mg/500 mL or a continuous infusion norepinephrine 3–10 mL/hour of a 2 mg/50 mL solution was used per clinical routine in our institution. General anaesthesia was induced and maintained per routine as intraoperative bispectral index value of 40–60. Fluid administration and blood transfusion were also per clinical judgement. Resumption of chronic anti-hypertensive medications was delayed until the third postoperative day unless deemed necessary to treat hypertension or for another clinical indication.
In patients assigned to routine pressure management, routinely used ACEIs and ARBs were given on the morning of surgery if deemed appropriate by the attending anesthesiologist. Vasopressors, as above, were used per the attending clinician’s discretion. General anaesthesia was induced and maintained per routine as intraoperative bispectral index of 40–60. Fluid administration and blood transfusion were also per clinical judgement. Intraoperative pressure management was per routine. As usual, chronic anti-hypertensive medication was restarted shortly after surgery unless contraindicated by hypotension.
Blinding
Randomisation and group assignment were performed by an investigator (KL) who did not participate in perioperative care or data collection. Anesthesiologists who were responsible for anaesthetic management were not involved in trial follow-up. Investigators (ZH, WL) who performed postoperative follow-up and patients were masked to study group assignment. The trial was thus assessor and patients blinded.
Data collection
The required data was collected by trained research staff, recorded in paper-based case report forms and then stored into Excel digital forms. Assessors will conduct the follow-up procedures in person.
Measurements
Intraoperative pressures from the required arterial catheter were automatically recorded in our electronic anaesthesia records at 1 min intervals before anaesthesia induction. Typically, postoperative pressures were measured oscillometrically at 8-hour intervals in surgical ward.
For perfusion-related complications, we considered a collapsed (one or more) composite of myocardial injury, stroke, non-fatal cardiac arrest, Stage 2–3 AKI defined by the creatinine component of the Kidney Disease: Improving Global Outcomes definition, deep or organ-space infection, sepsis and all-cause mortality within 30 days of surgery. We required high-sensitivity troponin I and creatinine preoperatively and daily for the initial three postoperative days.
MINS was defined as troponin I exceeding the local 99th percentile (0.04 ng/mL).13 Strokes were detected based on clinical symptoms and required radiographic evidence consistent with new-onset cerebral ischaemic or haemorrhagic injury. Delirium was assessed between 07:00 and 10:00 and again between 17:00 and 20:00 by 3D-CAM for the initial four postoperative days while patients remain hospitalised,14 with any positive assessment being considered evidence of delirium.
Primary outcomes
The first co-primary outcome was the fraction of time when intraoperative MAP was >85 mm Hg, intraoperative area (time integral) of MAP >85 mm Hg and intraoperative area (time integral) of MAP <65 mm Hg.
The second co-primary outcome was postoperative blood pressure management, characterised by the time routine antihypertensive medications were restarted after surgery.
Secondary outcomes
The secondary feasibility outcome measures were time-weighted average (TWA) intraoperative MAP, cumulative minimum MAP for 10 min, average postoperative systolic blood pressure (SBP) and mean of the lowest three postoperative SBPs. Cumulative minimum MAP for 10 min was calculated as the lowest MAP, at or below which a patient’s MAP was sustained for at least 10 min during the surgery. Post-hoc, we also defined the measures intraoperative area over MAP >80 mm Hg and area of MAP <60 mm Hg as additional secondary outcomes.
The exploratory efficacy outcome measures were: (1) perfusion-related complications within 30 days of surgery and (2) postoperative delirium within the first four postoperative days.
Data and sample storage
All relevant clinical trial materials will be saved for at least 3 years after termination of the trial. The investigators and statistician have access to the entire data set.
Data monitoring
The trial was coordinated by an executive committee, but there was not an external Data and Safety Monitoring Board.
Protocol changes
No protocol changes were made during the trial or before trial data were accessed.
Power calculation
The study enrolled 40 patients in each treatment group. The statistical analysis plan was finalised after patients were enrolled, but before data were accessed. As this was a feasibility trial, our primary goal was to assess implementation of the protocol. We nonetheless estimate how many patients were required to give us a reasonable sense of dispersion that would be the basis for a subsequent full trial.
The fraction of time spent above 85 mm Hg was not expected to be normally distributed and instead, assumed to have a Beta(5, 5) distribution. Using the above sample size, the CI half-width for estimating the mean would be 0.05, which was deemed to be sufficiently precise. The observed CI widths were 0.01.
Area of MAP <65 mm Hg is typically heavily skewed and not normally distributed. Using the same sample size as above, we assumed that area of MAP <65 mm Hg would be distributed as Gamma (0.25, 0.1) which would give us a CI half-width of 0.73. This was deemed to be sufficiently precise for the estimation of the mean area of MAP <65 mm Hg. The observed CI half-widths were 8.6 (reference group) and 0 (treatment group).
Statistical analysis
Balance on baseline characteristics was assessed using absolute standardised difference (ASD), which is defined as the absolute difference in means, ranks, or proportions divided by the pooled SD. Groups were considered to be imbalanced with respect to a baseline characteristic when ASD exceeded 0.44 [1.96*sqrt(1 /n1+1 /n2)].15
We primarily evaluated the effect of tight blood pressure control on the fraction of time when intraoperative MAP exceeded 85 mm Hg using a t-test. Wilcoxon rank-sum tests were used to evaluate the effect of tight perioperative BP control on intraoperative area of MAP >85 mm Hg, intraoperative area of MAP <65 mm Hg, and time to restart routine antihypertensive medications after surgery.
We also evaluated the effect of tight blood pressure control on intraoperative area of MAP >80 mm Hg, and intraoperative area of MAP <60 mm Hg using Wilcoxon rank-sum tests. We used two-sided, two-sample t-tests to evaluate the effect of blood pressure control on TWA intraoperative MAP, cumulative minimum MAP for 10 min, TWA SBP and mean of the lowest three postoperative SBPs. On an exploratory basis, we used log-binomial models to evaluate a collapsed composite of perfusion-related complications, and postoperative delirium.
All analyses were conducted using R V.4.0.2.
Patient and public involvement
There was no public or patient involvement.
Results
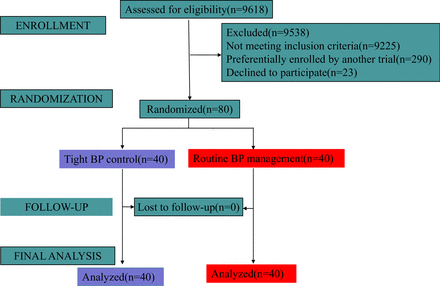
Between 23 May 2021 and 29 September 2021, 9618 cases were screened, and 393 were deemed eligible. Two-hundred and ninety cases were preferentially enrolled by another trial. A total of 103 patients were approached, and 80 consented. Forty patients were randomised to tight pressure management and 40 were randomised to routine pressure management. No patients withdrew before hospital discharge. Our CONSORT flow diagram is presented in figure 1. Three patients assigned to tight pressure management were lost between discharge and the 1-month follow-up assessment. There were thus 80 patients included in the primary analysis, and 77 in the 1-month analysis. The last patient follow-up was completed on 1 November 2021.
CONSORT flow diagram. Patient flow through stages of the trial. BP, blood pressure.
Patient demographic and baseline characteristics are presented in table 1. Only diastolic blood pressure and surgery type were imbalanced, with ASD >0.44. The median (Q1, Q3) blood loss was 175 (100, 300) mL in the tight perioperative BP control group and 50 (50, 200) mL in the routine BP management group.
Patient baseline and demographic characteristics
The mean (SD) fraction of time with intraoperative MAP exceeding 85 mm Hg (ie, time when MAP >85 mm Hg divided by total surgery duration) was 0.52 (0.25) in patients assigned to routine blood pressure management and 0.87 (0.15) in those assigned to tight control. The estimated absolute difference in the mean fraction of time between tight and routine blood pressure management was 0.35 (95% CI: 0.26 to 0.44).
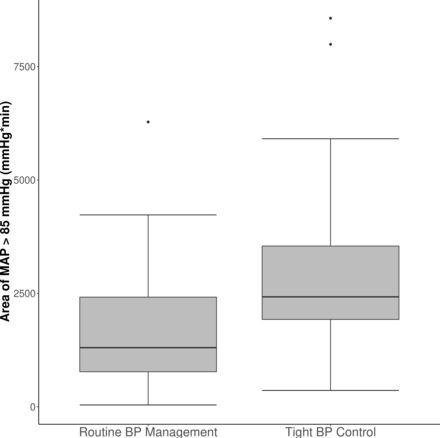
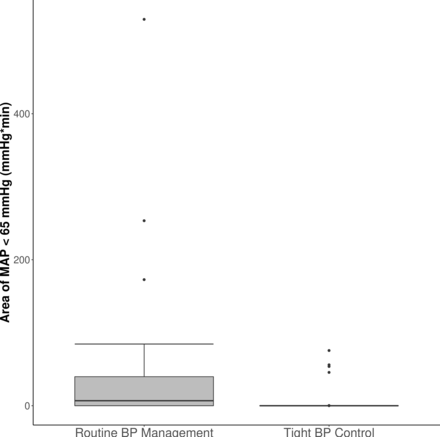
The median (Q1–Q3) intraoperative area of MAP >85 mm Hg was 1303 (772–2419) mm Hg*min in patients assigned to routine perioperative BP control and 2425 (1926–3545) mm Hg*min in those assigned to tight perioperative BP management (figure 2). Tight blood pressure control increased area of MAP >85 mm Hg by 1102 (95% CI: 596 to 1608) mm Hg*min. Similarly, for intraoperative area of MAP <65 mm Hg, the median (Q1–Q3) was 7 (0–40) mm Hg*min with routine pressure management, and 0 (0–0) mm Hg*min with tight control (figure 3). Tight pressure control thus reduced exposure to MAP <65 mm Hg by 6 (95% CI: 2 to 15) mm Hg*min. In the routine pressure management group, 40% (n=16) of the patients experienced hypotension (any time below 65 mm Hg) with a median (Q1–Q3) duration of 6 (2–9) min compared with 10% (n=4) in the tight blood pressure control group, with a median duration of 5 (4–6) min (online supplemental figure 1).
Supplemental material
Area of intraoperative MAP >85 mm Hg by blood pressure management group. The lower and upper edges of the box correspond to 25th and 75th percentile, respectively, with the central line representing the median; whiskers extend to 1.5 times the IQR from the edges of the box; data beyond the whiskers are outliers and plotted individually. BP, blood pressure; MAP, mean arterial pressure.
Area of intraoperative MAP <65 mm Hg by blood pressure management group. The lower and upper edges of the box correspond to 25th and 75th percentile, respectively, with the central line representing the median; whiskers extend to 1.5 times the IQR from the edges of the box; data beyond the whiskers are outliers and plotted individually. BP, blood pressure; MAP, mean arterial pressure.
The median (Q1, Q3) intraoperative area of MAP >80 mm Hg was 1917 (1249–3169) mm Hg*min in patients assigned to routine BP control and 3355 (2549–4417) mm Hg*min in the patients assigned to tight BP control. The area of MAP >80 mm Hg was thus 1335 (95% CI: 667 to 1928) mm Hg*min greater in patients assigned to tight pressure control. For intraoperative area of MAP <60 mm Hg, the median was 0 (0–6) mm Hg*min with routine pressure management and 0 (0–0) mm Hg*min with tight pressure control, for an estimated treatment effect of 0 (95% CI: 0 to 0) (table 2).
Summary of analysis results
The mean difference in the intraoperative TWA MAP between tight and routine pressure management was 12 (95% CI: 8 to 16) mm Hg, and the difference in cumulative intraoperative minimum MAP sustained for 10 min was 14 (95% CI: 11 to 18) mm Hg (online supplemental figure 2; table 2).
Supplemental material
Antihypertensive medications were restarted 2 (95% CI: 1 to 3) days later in patients assigned to tight blood pressure control. The difference (95% CI) in postoperative mean SBP between tight and routine pressure management was 5 (−1 to 11) mm Hg, and the difference in mean of the lowest three postoperative SBP measurements was also 5 (−1 to 11) mm Hg (online supplemental figure 3; table 2).
Supplemental material
The incidence of perfusion-related complications was 12% in both groups and the relative risk was estimated to be 1.0 (0.3–3.3). No delirium was detected in either group. No severe adverse events were attributed to the study.
Discussion
Intraoperative blood pressure management was well maintained in patients assigned to tight control titrated with norepinephrine intravenous infusion, and MAP exceeded the target of 85 mm Hg in 87% of the time. Furthermore, the average of the lowest MAPs sustained for 10 min in the tight group was 85 mm Hg, indicating that MAPs in these patients were only rarely and transiently less than 85 mm Hg. Intraoperative pressures also exceeded 85 mm Hg about half the time in patients assigned to routine management which is unsurprising since hypertension was an inclusion criterion for the trial.
Because patients cannot be randomised to hypotension, the more important question is the extent to which pressures were <65 mm Hg, which is thought to be the intraoperative harm threshold, with routine management. There was almost no hypotension in patients assigned to tight control whereas the median area <65 mm Hg was 7 mm Hg*min in those with routine management. Furthermore, the lowest MAPs sustained for 10 min in patients assigned to routine management was 71 (9) mm Hg, and about 40% of patients had lowest sustained pressures ≤65 mm Hg. There were thus distinct differences in MAPs in the two management groups, with one having virtually no hypotension and the other often experiencing mean pressures ≤65 mm Hg.
Antihypertensive management was successful with the mean restart day being 1 with routine management and 4 with tight management, corresponding to a difference of 2 (95% CI: 1 to 3) days which is a clinically meaningful difference. However, antihypertensive management had little effect on postoperative SBPs, with the lowest three measurements differing by only 5 mm Hg. Furthermore, the average lowest measurements exceeded 120 mm Hg which is well above proposed postoperative harm threshold defined either by an MAP of 75 mm Hg16 or an SBP of 90 mm Hg.5 These results suggest that antihypertensive management should not be included in the full trial.
Previous studies report considerably more postoperative hypotension than we observed. For example, Liem reported that 2 cumulative hours below threshold of 60 mm Hg occurred in 8% patients and 4 continuous hours less than 75 mm Hg occurred in 48% patients.16 Khanna and colleagues similarly reported that 63% of patients experienced an MAP ≤75 mm Hg within 48 hours after surgery, and that 22% experienced MAP ≤65 mm Hg.17 However, there were two important differences between our trial and previous observational reports. The first is that both previous reports were based on continuous non-invasive blood pressure monitoring rather than oscillometric assessments at 8-hour intervals. Continuous monitoring will obviously detect more hypotension than intermittent monitoring. Furthermore, continuous non-invasive monitors are not well validated and may at times generate false low values. More frequent postoperative measurements would be helpful in a full trial. The second important difference between current and previous results is that enrolment in our feasibility trial was restricted to patients taking anti-hypertensive medications, and thus having a diagnosis of hypertension. It is understandable that hypertensive patients would have less hypotension than a general surgical population.
Only 10 of 80 patients experienced our composite outcome of major perfusion-related complications, evenly split between the treatment groups. With so few events, the (lack of) difference between the groups is non-informative. Curiously, no delirium was observed in our 80 patients. The incidence of delirium after non-cardiac surgery varies widely, but is probably now lower than previously reported.18 19 Delirium incidence also clearly depends strongly on age, with the incidence increasing markedly in patients older than 65 years.9 The average age in our patients was 67 years which is relatively young which may have contributed to lack of observed delirium.
The major limitation of our trial is that it was conducted in a single centre whereas our planned full trial will involve dozens of centres around the world. Our results demonstrating that intraoperative pressure and postoperative antihypertensive management is feasible does not mean that it will prove practical at all trial sites. Because the trial was only powered for feasibility and pressure management, the incidence of hard outcomes (based on only 10 events) is essentially non-informative. Patients assigned to routine care had slightly lower diastolic blood pressures, 80 versus 86 mm Hg. However, this small difference seems unlikely to have much influenced our conclusions.
Conclusion
We achieved substantial separation of intraoperative MAPs. Similarly, we were able to control restarting antihypertensive medications per protocol—although doing so had relatively little effect on postoperative SBPs. Furthermore, the requirement that all patients have chronic hypertension resulted in relatively high intraoperative and postoperative pressures. Consequently, we amended the protocol for the full trial to include patients without chronic hypertension and no longer specify postoperative antihypertensive management. The full trial appears feasible and remains well warranted.
Data availability statement
Data are available upon reasonable request. The investigators and statistician have access to the whole data set. Data will be shared collaboratively with external parties with the approval of the Executive Committee and appropriate data-use agreements.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved. The protocol was approved by The Ethics Committee of the China-Japan Union Hospital of Jilin University, Changchun, China (Chairperson Prof. Songyan Liu) on 30 November 2020 (Approval number: 20201120). Participants gave informed consent to participate in the study before taking part.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors KL was the principal investigator and helped design the protocol, applied for ethics approval, registered the trial, assigned groups and drafted the manuscript. ZH and WL helped collect data. KS was responsible for data analysis. DS designed the protocol and provided overall trial guidance. All authors reviewed and revised the manuscript. KL is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests DS is a consultant for Edwards Lifesciences (Irvine, California). He serves on advisory boards and has equity interests in Calorint (Philadelphia, Philadelphia), TransQtronics (Philadelphia, Philadelphia), the Health Data Analytics Institute (Boston, Massachusetts), Medasense (Tel Aviv, Israel), Serenno (Tel Aviv, Israel) and Perceptive Medical (Newport Beach, California).
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.