Article Text
Abstract
Objectives Determine the prevaccination healthcare impact of COVID-19 in patients with systemic lupus erythematosus (SLE) in England.
Design Retrospective cohort study of adult patients with SLE from 1 May to 31 October 2020.
Setting Clinical Practice Research Datalink (CPRD) Aurum and Hospital Episode Statistics (HES) databases from general practitioners across England combining primary care and other health-related data.
Participants Overall, 6145 adults with confirmed SLE diagnosis ≥1 year prior to 1 May 2020 were included. Most patients were women (91.0%), white (67.1%), and diagnosed with SLE at age <50 (70.8%). Patients were excluded if they had a COVID-19 diagnosis before 1 May 2020.
Primary and secondary outcome measures Demographics and clinical characteristics were compared. COVID-19 severity was determined by patient care required and procedure/diagnosis codes. COVID-19 cumulative incidence, hospitalisation rates, lengths of stay and mortality rates were determined and stratified by SLE and COVID-19 severity.
Results Of 6145 patients, 3927 had mild, 1288 moderate and 930 severe SLE at baseline. The majority of patients with moderate to severe SLE were on oral corticosteroids and antimalarial treatments. Overall, 54/6145 (0.88%) patients with SLE acquired and were diagnosed with COVID-19, with 45 classified as mild, 6 moderate and 3 severe COVID-19. Cumulative incidence was higher in patients with severe SLE (1.4%) compared with patients classified as mild (0.8%) or moderate (0.8%). Ten COVID-19-specific hospital admissions occurred (n=6 moderate; n=4 severe). Regardless of COVID-19 status, hospital admission rates and length of stay increased with SLE severity. Of 54 patients with SLE diagnosed with COVID-19, 1 (1.9%) COVID-19-related death was recorded in a patient with both severe SLE and severe COVID-19.
Conclusions SLE severity did not appear to impact COVID-19 outcomes in this study. The COVID-19 pandemic is evolving and follow-up studies are needed to understand the relationship between COVID-19 and SLE.
- COVID-19
- HEALTH ECONOMICS
- RHEUMATOLOGY
- GENERAL MEDICINE (see Internal Medicine)
- INFECTIOUS DISEASES
- PUBLIC HEALTH
Data availability statement
Data are available upon reasonable request. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- COVID-19
- HEALTH ECONOMICS
- RHEUMATOLOGY
- GENERAL MEDICINE (see Internal Medicine)
- INFECTIOUS DISEASES
- PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study provided unique insight into the outcomes of COVID-19 for patients with systemic lupus erythematosus (SLE) before the availability of COVID-19 vaccines.
Due to the nature of a database study, there were limitations in the data captured in the system.
The number of diagnosed COVID-19 cases was low in patients with SLE.
The information about secondary care prescriptions in this population was limited.
Introduction
Since December 2019, infection with SARS-CoV-2, the causative agent of COVID-19, has caused significant morbidity and mortality worldwide, with over 6.6 million deaths as of December 2022.1 2 Case fatality rate of SARS-CoV-2 infection was estimated from data published by the WHO, as of December 2022, to be 0.8% in the United Kingdom and 1.0% globally.1 Case fatality rates can vary substantially according to viral strain and pathogenicity and across countries and patient subgroups,3–8 with personal health status, including age and underlying diseases, significantly impacting the risk and prognosis of SARS-CoV-2 infection.7 COVID-19 symptoms and disease state can vary in severity from mild flu-like symptoms to severe life-threatening disease,9 with critical COVID-19 disease, leading to acute respiratory distress syndrome, sepsis and septic shock, cardiac disease and thromboembolic events such as pulmonary disease and multiple organ failure.10 11 Overall, the wide spectrum of symptoms and multisystem nature of this disease continue to make COVID-19 a global threat, especially to high-risk groups.11 12
Systemic lupus erythematosus (SLE) is a heterogenous, chronic, autoimmune disease that presents as a range of clinical manifestations across organ systems, with variable severity, disease course and prognosis.13 14 Both the innate and adaptive immune responses are dysregulated in patients with SLE,14 15 leading to the production of pathogenic auto-antibodies that cause inflammation and tissue damage.16 SLE disease activity is commonly controlled with immunosuppressive therapies; therefore, patients may be more susceptible to infection.13 Both SLE disease activity and prolonged glucocorticoid use contribute towards progressive organ damage.17–19
SLE and COVID-19 are both complex, multisystem diseases. During the early stages of the pandemic, the British Society for Rheumatology (BSR) classified SLE patients as at normal, moderate or high risk of severe illness from COVID-19 depending on their disease symptoms and treatment20; however, it has been difficult to determine whether patients with SLE are more susceptible to SARS-CoV-2 infection or severe presentations of COVID-19. Additionally, there is limited evidence if SLE treatments confer a protective or detrimental effect on SARS-CoV-2 infection in patients with SLE.21 While standard therapies and organ damage may make patients with SLE more susceptible to severe COVID-19, it is unclear what the full extent of COVID-19 disease complications may be for patients with SLE.22 23
Our study aimed to examine COVID-19 impact on adult patients with SLE in England from May 2020 to October 2020, prior to the start of the COVID-19 vaccination programme and the emergence of key SARS-CoV-2 variants of concern, such as the delta variant. Data from the linked Clinical Practice Research Datalink (CPRD) Aurum, Hospital Episode Statistics (HES) and Office for National Statistics (ONS) death registry databases were used to determine the incidence of COVID-19 among patients with SLE, stratified by severity and the demographic and clinical characteristics of patients with SLE who were diagnosed with COVID-19. We also determined hospitalisation rate, length of stay and mortality rate of patients with SLE, with and without COVID-19, stratified by both SLE and COVID-19 severity.
Methods
Study design
This was an observational, retrospective cohort study of adult patients with SLE in England between 1 May 2020 and 31 October 2020. This timeframe was selected because SARS-CoV-2 testing capabilities in England were expanded beyond pilot testing of critical key workers and patients with COVID-19 in April 2020.24 In early December 2020,25 vaccination against COVID-19 began in England, and, therefore, the study cut-off date of 31 October 2020 was selected to avoid capturing the interaction of COVID-19 vaccinations with COVID-19 disease among the SLE population. A schematic of the study design is shown in figure 1.
Schematic of study design and criteria for patient selection. Patients were stratified by SLE severity within the 12-month baseline period (May 2019 to May 2020). All patients were required to have valid data to be considered for evaluation in the follow-up period and to be considered at-risk of COVID-19 within the study. CPRD, Clinical Practice Research Datalink; HES, Hospital Episode Statistics; ID, index date; SLE, systemic lupus erythematosus.
Datasets
The study used electronic medical record data from the CPRD Aurum database, which collects deidentified patient data from a network of general practitioners across England and links primary care data to a range of other health-related data, providing a longitudinal health data set broadly representative of geographical coverage, area-level deprivation, age and sex in England.26 The CPRD Aurum database encompasses 60 million patient lives, with approximately 18 million patients currently registered.27 CPRD Aurum records were linked to the HES database, which records information on inpatient admissions, outpatient appointments and accident and emergency attendances in England.26 Relevant hospital admissions, including admission of patients with SLE who had COVID-19 as the primary diagnosis, were identified. CPRD records were also linked to the ONS database, which records annual mortality data registered by age, sex and selected underlying cause of death.28 29 Consent for sharing patient data with CPRD Aurum was provided by clinical practices, with individual-level opt-out choice offered and implemented on request.26 Further information regarding these data sets is shown in online supplemental table 1.
Supplemental material
Population
A flowchart describing patient selection procedures is shown in online supplemental figure 1. Eligible patients were aged 18 years or older presenting at primary or secondary care with one or more diagnosis codes for SLE, determined by database codes in primary care, or an International Classification of Diseases (ICD-10) code in secondary care. The first recorded diagnosis (the index diagnosis) of SLE was required to be prior to 1 May 2019. SLE was confirmed by inclusion of at least one subsequent diagnosis of SLE following the index diagnosis. Patients were required to have valid data available beyond 1 May 2020.
Patients were excluded if they had drug-induced, cutaneous or discoid lupus, or if they did not have a ‘definitive code’ anywhere in their CPRD record or HES to confirm diagnosis. Patients were also excluded if a diagnosis of COVID-19 was recorded prior to the beginning of the study observation period on 1 May 2020.
Disease severity classifications
The main variables calculated for each patient were SLE severity, determined at the beginning of the observation period, and COVID-19 severity, where applicable.
SLE disease severity
SLE disease severity subgroups (severe, moderate or mild) were determined based on published classification criteria,30 previously used in a retrospective cohort analysis study in the UK.31 Specifically, patients were classified as having severe SLE if they had a prescription of cyclophosphamide or rituximab or oral glucocorticoids at a dosage of ≥60 mg/day prednisone equivalent and had ≥1 ICD-10 code for diagnosis of severe renal, cardiovascular, hepatic, gastrointestinal, neurological, ocular or other comorbidities. Patients were classified as having moderate SLE if they were prescribed immunosuppressants (excluding cyclophosphamide) or oral glucocorticoids at a dosage of 7.5 mg/day to <60 mg/day prednisone equivalent, and if they had ≥1 ICD-10 code for diagnosis of moderate renal, cardiovascular, hepatic, gastrointestinal, neurological, ocular or other comorbidities. Patients whose SLE was not considered moderate or severe were defined as mild. SLE severity was evaluated from 12 months prior to study entry (1 May 2019 to 1 May 2020), and the highest severity observed within this period was recorded.
COVID-19 diagnosis and severity
Diagnosis of COVID-19 was identified using CPRD database codes in primary care and the HES ICD-10 code in secondary care. Patients with a confirmed COVID-19 diagnosis were stratified based on COVID-19 severity.
COVID-19 severity was determined using the following definitions: COVID-19 was deemed severe if, in the same admission, as a new COVID-19 diagnosis or with COVID-19 in the primary diagnosis position, the patient required critical/intensive care in any episode during admission, and/or required mechanical ventilation (Office of Population Censuses and Surveys−4 procedure code), and/or experienced shock or sepsis (ICD-10 diagnosis codes) and/or experienced organ failure not previously coded (heart, lung, kidney, liver) (ICD-10 code). COVID-19 was deemed moderate if the patient was hospitalised with a new COVID-19 diagnosis but did not meet the severe criteria. COVID-19 was deemed mild if the patient had any new COVID-19 diagnoses outside of secondary care.
Study outcomes
Outcomes were evaluated in all identified patients with SLE overall, and in mild, moderate and severe SLE subgroups. The total number and cumulative incidence of COVID-19 infections per calendar month from 1 May 2020 to 31 October 2020 were calculated and stratified by COVID-19 severity. Patient demographics and clinical disease characteristics with respect to both SLE severity and COVID-19 severity were also compared.
Among patients with SLE who developed COVID-19, the following clinical outcomes were evaluated for each SLE subgroup and COVID-19 severity group: age at COVID-19 diagnosis, acute case fatality rate of COVID-19 (defined as a patient death within 28 days of an initial COVID-19 diagnosis and reported within the ONS death registry over the total number of COVID-19 cases in the target population), COVID-19-specific hospitalisation rate and length of stay, all-cause hospital admission rate per 1000 patients and lengths of stay (bed days) among COVID-19 severity groups, including those without a COVID-19 diagnosis, and number of patients with respiratory distress, organ failure, or pneumonia and of patients requiring oxygen therapy or mechanical ventilation.
Statistical analysis
This was a descriptive study and, therefore, no comparative statistical analyses were planned or performed. Descriptive statistics for the study population were calculated, including total numbers of patients, clinical and demographic profiles and length of time patients with SLE were followed since diagnosis and inclusion within the study. Cumulative incidence of COVID-19 infections was determined monthly. Right censoring was used for patients who had no record of outcomes by the end of the 6-month study period. Left censoring was mitigated through record review for at least 10 years prior to the index date of 1 May 2020.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination of this research.
Results
Demographics and disease characteristics of patients with SLE
Overall, 6145 patients were included for analysis, with 3927 defined as having mild SLE, 1288 with moderate SLE and 930 with severe SLE. Demographics and SLE disease characteristics at the index date, both overall and according to SLE disease severity, are shown in table 1. The majority of patients with moderate to severe SLE were on oral corticosteroids and antimalarial therapy, according to primary care prescription data. A total of 4350/6145 (70.8%) patients were diagnosed with SLE at age <50 years, with a mean 42.2 years of age (SD 14.2) at diagnosis. Mean age (SD) of diagnosis was similar across SLE disease severity subgroups, with 42.0 (13.9) years for mild, 41.7 (14.9) years for moderate and 43.5 (14.7) years for severe SLE. The majority of patients were female (91.0%), and most patients were White (67.1%), with smaller proportions of patients of Black (11.7%) or Asian (10.2%) race. Overall, 80.0% of patients had a low Charlson comorbidity score (<2). The most prevalent comorbidities were hypertension (19.1%), asthma (17.9%), history of pneumonia (14.7%) and diabetes (12.4%).
Demographics and disease characteristics at index of patients with SLE according to SLE severity
Incidence of COVID-19 in patients with SLE
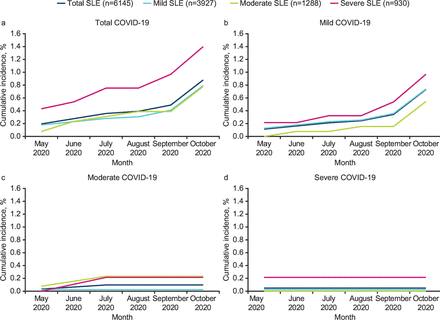
From 1 May 2020 to 31 October 2020, 54 (0.88%) of the 6145 patients with SLE were diagnosed with COVID-19. Of these 54 cases, 45 (83.3%) were classified as mild COVID-19, 6 (11.1%) were moderate and 3 (5.6%) were severe. Overall cumulative incidence of COVID-19 over the 6-month observation period and according to SLE severity subgroup is shown in figure 2. Cumulative incidence of total COVID-19 cases rose more steeply in patients with severe SLE compared with patients classified with mild or moderate SLE (figure 2). This difference was driven predominantly by an increase in mild COVID-19 cases in patients with severe SLE.
Cumulative incidence of COVID-19 diagnoses over the 6-month evaluation period according to SLE severity. No comparative inferential statistical analyses were performed; cumulative incidence of COVID-19 diagnoses across SLE subgroups was evaluated with descriptive statistics only. SLE, systemic lupus erythematosus.
Demographics and disease characteristics of patients with and without COVID-19 are shown in table 2. Compared with the 6091 patients with SLE without COVID-19, the 54 patients with COVID-19 were slightly older (mean age 45.2 vs 42.1 years), with similar body mass indices (mean 25.9 vs 26.3), and a similar proportion was women (92.6% vs 91.0%). A greater proportion of patients with versus without COVID-19 had a Charlson comorbidity score of ≥2 (33.3% vs 19.9%) and had comorbidities, including diabetes (24.1% vs 12.3%), hypertension (27.8% vs 19.0%), history of pneumonia (25.9% vs 14.6%), asthma (22.2% vs 17.9%), and history of myocardial infarction (11.1% vs 3.5%). Of the 54 patients diagnosed with COVID-19, 31 had mild, 10 had moderate and 13 had severe SLE (table 2).
Demographics and disease characteristics at index of patients with SLE with and without COVID-19 diagnosis
There was a trend towards patients with severe SLE also having a severe COVID-19 diagnosis (patients with severe SLE made up 9/45 (20.0%) of mild, 2/6 (33.3%) of moderate and 2/3 (66.7%) of severe COVID-19 cases); however, there were small numbers of patients who had severe COVID-19 (n=3) (online supplemental figure 2).
Clinical outcomes
A summary of clinical outcomes is found in table 3. The mean age (SD) at COVID-19 diagnosis was 55.8 (17.8) years overall for all patients with SLE, 53.7 (16.2) years in patients with mild SLE, 69.1 (18.9) years in patients with moderate SLE and 54.1 (15.2) years in patients with severe SLE.
Clinical outcomes in patients with SLE with and without COVID-19 according to SLE severity
Hospitalisations
Among the 54 patients with SLE and COVID-19, there were 10 recorded COVID-19-specific hospitalisations, as defined by diagnostic codes in the primary diagnostic position in the same admission as was documented in the HES database (table 3). Note that one patient can be hospitalised multiple times. Of these hospitalisations, six were for moderate COVID-19 (six patients) and four were for severe COVID-19 (three patients). Of the six patients hospitalised with moderate COVID-19, one had mild, three had moderate and two had severe SLE; the mean (SD) length of stay for these patients was 10.2 (6.2) days. Of the three patients hospitalised with severe COVID-19, one patient with severe SLE was hospitalised once, one patient with severe SLE was hospitalised two times, and one patient with mild SLE was hospitalised once; the mean (SD) length of stay was 18.0 (18.0) days. In total, there were 2152 all-cause hospital admissions among the SLE cohort during the observation period, 96 of which occurred in patients diagnosed with COVID-19.
The all-cause hospital admission rate per 1000 patients increased with severity of SLE regardless of COVID-19 status (from 158 for mild SLE to 1125 for severe SLE in patients without COVID-19, and from 194 for mild SLE to 6385 for severe SLE in patients with COVID-19) (table 3). The all-cause mean hospital length of stay also increased with severity of SLE regardless of COVID-19 status (from 3.0 days for mild SLE to 6.4 days for severe SLE in patients without COVID-19 and from 0.3 days for mild SLE to 16.0 days for severe SLE in patients with COVID-19) (table 3).
Deaths
There were 45/6091 (0.74%) deaths among patients with SLE without a COVID-19 diagnosis and 2/54 (3.7%) deaths among patients with SLE who were diagnosed with COVID-19 (table 3). Only one death was deemed related to COVID-19 and occurred in a patient diagnosed with SLE more than 10 years prior to this study, who was classified as having severe SLE and had multiple additional comorbidities. During this period, the patient received prescriptions of prednisone, methotrexate and rituximab. Death occurred during an admission for COVID-19. The second death caused by acute myocardial infarction occurred in a patient classified with mild SLE and mild COVID-19. Overall, the acute COVID-19 case fatality rate was 19 per 1000 patients with SLE.
Discussion
We retrospectively evaluated the incidence and outcomes of COVID-19 among a large SLE cohort in England prior to the advent of vaccination from 1 May 2020 to 31 October, 2020. We found few cases of COVID-19 in this cohort over this time period, and among those, a small number were severe. Interestingly, the cumulative incidence of total COVID-19 cases appeared greater in patients with severe SLE as compared with mild or moderate SLE, although this was driven predominantly by mild COVID-19 cases.
The overall incidence of COVID-19 in patients with SLE during the 6-month observation period was low (0.9%). In the general population, COVID-19 incidence from 1 May 2020 to 31 October 2020 in England was approximately 1.3%.32 33 Low incidence of COVID-19 among patients with SLE could have been due to low testing rates, leading to underestimates of infection rates during this timeframe in combination with public health precautions used to prevent the spread of SARS-CoV-2.34 35
Our study identified some differences between the demographic and clinical characteristics of patients with SLE who were diagnosed with COVID-19 and those who were not, including older age and the prevalence of comorbidities (diabetes, hypertension, history of pneumonia, asthma and history of myocardial infarction). The demographic and clinical differences were in line with previously identified risk factors for severe COVID-19 disease outcomes in the non-SLE population.12 36 Although, to our knowledge, studies linking SLE disease activity and susceptibility for being infected with SARS-CoV-2 have not yet been published, previous studies have shown an association between SLE severity and developing severe COVID-19.23 Furthermore, SLE disease activity has been previously identified as a risk factor for serious non-SARS-CoV-2 infections (eg, urinary tract infection, lower respiratory tract infection) and, conversely, attainment of low disease activity state was protective against serious infections.37
The BSR considers patients with SLE as at high risk of developing severe COVID-19 disease if they have poorly controlled disease/recent flares, are receiving high dosages of glucocorticoids or are receiving certain immunosuppressive drugs.20 Patients classified as having severe SLE in this study would have been categorised as high risk during the pandemic and, therefore, would have been advised by the NHS to ‘shield’ and be less exposed to COVID-19 infection.20 38 Our findings suggest that this shielding did not completely circumvent the potentially increased COVID-19 infection risk for some SLE patients. Notably, testing was not available for the general population in England until 2021.39 It would be difficult to extrapolate on the prioritisation of SLE patients in terms of COVID-19 testing. However, for patients to receive care in hospitals, a test would have been required.40 Thus, in line with the objective of this study to examine the healthcare impact of COVID-19 in patients with SLE, COVID-19 testing would have been an assumed step for hospitalised patients based on NHS guidance at the time40 and was captured in our dataset.
During the height of the COVID-19 pandemic, the NHS prioritised treatment of patients with COVID-19 in hospitals, which led to patients with SLE receiving more care at home through phone consultations.41 42 This shift in SLE management may have led to prioritised hospital admissions for patients with SLE following a COVID-19 diagnosis. In this study, being diagnosed with COVID-19 at any severity was associated with an increased rate of subsequent all-cause hospitalisation at any time after COVID-19 diagnosis and prolonged length of stay in those admissions compared with not having COVID-19; however, only 10 of the 2152 hospital admissions were deemed COVID-19 related. The mortality rate of COVID-19 in patients with SLE was low, and there was only one COVID-specific death, which occurred in a patient with severe SLE.
Study limitations include that this is a database study and, therefore, analyses are limited by the type of data and extent to which said data are captured in the system. As such, we likely underestimated the incidence of positive COVID-19 cases in patients with SLE. There may have been a selection bias for patients who had valid data available beyond 1 May 2020, as individuals who acquired COVID-19 prior to this date and died were not included. Deaths, hospitalisations and diagnosis outside of England were also not captured in our data set. These limitations are partially alleviated by the inclusion of a large number of patients with SLE in this study who were previously deemed to be representative of the UK population (>6100 patients out of an eligible >7700 patients).43 44 Although the CPRD Aurum database covers 16.45% of practices in the UK,45 SLE is usually diagnosed by a rheumatologist or other specialists rather than in primary care.46 Furthermore, only 2.7% of all patients opted-out from sharing their clinical data for research purposes by September 2020.47 The use of HES to search for SLE diagnosis likely also provided a reliable picture of SLE incidence in hospitalised patients in England.
Additional limitations include that SLE severity classification criteria used in this study did not include detailed SLE severity classification and was instead based on patients’ prescribed medication and recorded ICD-10 codes for various comorbidities, which are challenging to capture completely in healthcare databases. There were significant limitations in capturing secondary care prescriptions in this data set, resulting in limited numbers of biologic, cyclophosphamide and glucocorticoid use and a possible underestimation of other SLE prescriptions. Patients were classified as having mild, moderate or severe SLE; however, a dichotomous classification comparing mild to moderate/severe disease may better capture clinically relevant disease activity in this heterogeneous condition.48 Furthermore, SLE severity was classified based on the highest severity status within the 12-month timeframe prior to study entry, and disease activity/treatment could change during this period. However, the highest severity was considered in order to look at the ‘worst case scenario’ in assessing SLE patients for the objectives in the study. Sample size was also low due to the small number of diagnosed COVID-19 cases among these patients with SLE. Overall, the data used in this study represent a ‘snapshot’ of time in the fast-moving landscape of the COVID-19 pandemic. This provided unique insight into this SLE population prior to the availability of COVID-19 vaccines and was both a strength and a limitation of the study.
Treatment recommendations and preventive strategies for COVID-19 have been evolving quickly, making it challenging to evaluate the risk of SLE and its therapies alone in the absence of vaccination or native infection. The population included in this analysis was vaccine-naïve; however, since the study period (May 2020 to October 2020), a large-scale vaccination scheme has been introduced in the UK.49 The start date was chosen due to the lack of widespread community testing in the UK prior to this date. Inclusion of COVID-19 diagnoses prior to this date would capture only the most severe hospitalised cases, underestimating the true incidence and overestimating severity of infections within this time period. Some patients with SLE were considered a high-risk group and were eligible to receive their first COVID-19 vaccination in the UK from February 2021.50 Findings of this study of vaccine-naïve patients with SLE may not be translatable to vaccinated patients with SLE as vaccines have changed the prognosis of COVID-19 in the UK51 but may be of interest in countries with lower vaccination rates and less controlled stages of the COVID-19 pandemic. Additionally, it is known that different SARS-CoV-2 variants have differing virulence characteristics,4 23 52 which could be impacted by SLE-related disease or treatment factors and further influenced by primary immunity acquired from native SARS-CoV-2 infection or prior vaccination.52 53 Therefore, studies such as the one presented here provide an important evaluation of COVID-19 in a prevaccination population of patients with SLE for future analysis to build on. Vaccination against COVID-19 is reported to be safe and efficacious in patients with SLE with minimal risk of flares, and continued analysis of COVID-19 vaccination data will be useful in understanding the long-term impact of vaccination in patients with SLE.23 54 55
Conclusions
In conclusion, analysis of this large retrospective cohort study of 6145 patients with SLE in England suggested that SARS-CoV-2 infection was more prevalent in patients with severe versus mild or moderate SLE; despite small group sizes, SLE severity did not appear to impact COVID-19 outcomes. Results from this study provide a unique snapshot into the outcomes of COVID-19 for patients with SLE in England during the prevaccine phase of the pandemic, when government-imposed safety measures were in place. Given the evolving nature of the COVID-19 pandemic, including changes in safety measures, vaccination rates, diagnostic methods and treatment options as well as the infectiousness and pathogenicity of new SARS-CoV-2 variants, follow-up studies are needed to fully understand the impact of COVID-19 on patients with SLE in other geographic regions over a longer period of time.
Data availability statement
Data are available upon reasonable request. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Ethics statements
Patient consent for publication
Ethics approval
This study used data that existed in an anonymised, structured format that contained no personal patient information. The study protocol was reviewed and approved by CPRD’s Independent Scientific Advisory Committee (application number 21_000327) on 9 March 2021. Linkage of datasets was performed using anonymised and pseudonymised patient identification codes and was undertaken by NHS Digital, following study protocol approval. The CPRD obtains research ethics approval annually for receiving and supplying patient data for public health research from the UK’s Health Research Authority Research Ethics Committee; no additional ethics approval is required for observational studies in public health research using CPRD Aurum data.
Acknowledgments
Data analysis was performed by Health IQ LTD. Writing assistance was provided by Kelly M. Hunter, PhD, of JK Associates Inc., part of Fishawack Health. This work was supported by funding from AstraZeneca.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors APJR, WJL, RNK, RT and HAS-F designed the research study. APJR, WL conducted the research. APJR, WJL, RNK, HAS-F and JW performed the analysis. APJR, WJL, RNK, RT, HAS-F, JW and KW contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. APJR is acting as guarantor.
Competing interests APJR, RNK, RT and HAS-F are employees of and stockholders in AstraZeneca. HAS-F is a stockholder of GlaxoSmithKline (GSK). KW has served as a consultant to AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Eli Lilly & Company, Galapagos, Gilead, GSK, Novartis, Pfizer, Roche, Regeneron, Sanofi, and Union Chimique Belge (UCB); and has received grant/research support from BMS and Pfizer.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.