Article Text
Abstract
Introduction Pneumonic-type primary pulmonary lymphoma (PPL) is often misdiagnosed as pneumonia in clinical practice. However, this disease requires different treatments, which calls for a correct diagnosis.
Materials and methods A total of 227 patients with pneumonic-type PPL (n=72) and pneumonia (n=155) from 7 institutions were retrospectively enrolled between January 2017 and January 2022. Clinical features (age, sex, cough, sputum, fever, haemoptysis, chest pain, smoking, weight loss and laboratory results (haemoglobin, white blood cell count, C reactive protein level and erythrocyte sedimentation rate)) and CT imaging characteristics (air bronchogram, bronchiectasis, halo sign, pleural traction, pleural effusion, lymphadenopathy, lesion maximum diameter and CT attenuation value) were analysed. Receiver operating characteristic curve analysis was performed for model construction based on independent predictors in identifying pneumonic-type PPL. In addition, we used a calibration curve and decision curve analysis to estimate the diagnostic efficiency of the model.
Results The patients with pneumonia showed a higher prevalence of sputum, fever, leucocytosis and elevation of C reactive protein level than those with pneumonic-type PPL (p=0.002, p<0.001, p=0.011 and p<0.001, respectively). Bronchiectasis, halo sign and higher CT attenuation value were more frequently present in pneumonic-type PPL than in pneumonia (all p<0.001). Pleural effusion was more commonly observed in patients with pneumonia than those with pneumonic-type PPL (p<0.001). Also, sputum, fever, elevation of C reactive protein level, halo sign, bronchiectasis, pleural effusion and CT attenuation value were the independent predictors of the presence of pneumonic-type PPL with an area under the curve value of 0.908 (95% CI, 0.863 to 0.942).
Conclusion Pneumonic-type PPL and pneumonia have different clinical and imaging features. These differential features could be beneficial in guiding early diagnosis and subsequent initiation of therapy.
- computed tomography
- radiation oncology
- diagnostic radiology
Data availability statement
Data are available upon reasonable request. The original contributions presented in the study are included in the article/online supplemental material. Further inquiries can be directed to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study reported on differential clinical and CT imaging features between pneumonic-type primary pulmonary lymphoma and pneumonia, which can help early diagnosis and treatment choice.
The study was a multicentre study with a relatively large sample size and good generalisability.
Detailed subtypes of lymphoma and pneumonia were not specifically studied.
Imaging features on contrast-enhanced CT were not studied in detail.
Background
Primary pulmonary lymphoma (PPL) is defined as clonal lymphoid proliferation confined to the lung without extrathoracic lymphoma at diagnosis or within the next 3 months.1 PPL is a rare but severe condition that accounts for only 0.4% of all lymphomas and approximately 0.5% of primary pulmonary tumours.2 The absence of typical clinical presentations makes PPL difficult to identify. The radiological findings of PPL on CT imaging can present as nodules, mass and consolidation,3 4 which are also the most common features.4 5 Yet, PPL presenting consolidation on CT can be easily misdiagnosed as pneumonia, which calls for a correct diagnosis, considering that PPL and pneumonia require different treatments.
Previous studies have assessed the clinical manifestation, imaging features, pathology, treatment and prognosis of PPL.6 7 Kawel et al2 tried to differentiate pulmonary aspergillosis and pulmonary lymphoma using CT. However, the sample size of previous studies was small, which may be due to the rarity of this condition and the lack of focus on the differential diagnosis of PPL presenting consolidation on CT. Additionally, numerous cases of lymphoma presenting as consolidation on CT mistaken for pneumonia have been reported,8–10 further confirming insufficient understanding between lymphoma and pneumonia. Some studies showed that discrimination between PPL presenting as consolidation and pneumonia may be beneficial in guiding early diagnosis, evaluation and initiation of therapy.2 11 12
In this study, we defined the pneumonic-type PPL based on image characteristics aiming to evaluate the differential clinical and imaging features between pneumonic-type PPL and pneumonia from seven institutions.
Materials and methods
Patients
We searched for radiology reports at seven institutions from January 2017 to January 2022. The following keywords were used: ‘lung lymphoma’, ‘lung cancer’, ‘infectious lesions’ and ‘pneumonia’ on CT scans of the lung. Inclusion criteria for pneumonic-type PPL were: (1) pathologically confirmed PPL by surgical resection, transbronchial lung biopsy or percutaneous lung biopsy within a week of CT scan and (2) presenting as consolidation on CT. Inclusion criteria for pneumonia were: (1) pathologically or clinically proven pneumonia and (2) presenting as consolidation on CT. Common exclusion criteria for both diseases were as follows: (1) poor images, (2) incomplete clinical data and (3) acquiring any antitumour or anti-inflammatory treatment before CT examination.
Two radiologists (XW and SZ with 25 and 12 years of experience in lung radiology, respectively) were responsible for the selection process at the different sites; any disagreements were resolved by discussion. To avoid recall bias, the two radiologists were not involved in the CT image analysis evaluation.
Demographic and clinical data including age, sex, cough, sputum, fever, haemoptysis, chest pain, smoking, weight loss and laboratory results (elevation of haemoglobin (>175 g/L for men and >155 g/L for women), leucocytosis (>10×109/L), C reactive protein level (>10 mg/L) and erythrocyte sedimentation rate (>27 mm/hour)) were collected from the medical records by a trained nurse (LW). The clinical variables before treatment were assessed. The time frame between blood results and imaging was within a day.
CT protocols
The details of CT parameters from seven institutions are shown in online supplemental table 1. All patients underwent non-enhanced CT scans.
Supplemental material
Supplemental material
Supplemental material
Image analysis
The pneumonic-type PPL was defined as consolidation along the lung lobe or segment on CT imaging. The CT imaging characteristics included air bronchogram (air-filled bronchi within lesions), bronchiectasis (dilatated bronchus through consolidation), halo sign (ill-defined ground-glass opacity around consolidation), pleural traction (linear structures connected between the lesion and pleura), pleural effusion, lymphadenopathy (hilar or mediastinal lymph nodes>1 cm in short axis diameter), lesion maximum diameter and CT attenuation value. The largest diameter of the lesion was measured at the largest section of the lesion on the transverse section. The CT attenuation values were acquired by using two 0.5 cm circular regions of interest for each lesion (avoiding blood vessels and airways within the lesion), and the averages of the values were recorded.
The CT characteristics before treatment were evaluated. All images were independently evaluated by two radiologists (SL and BJ with 7 and 9 years of experience in the field of thoracic imaging, respectively), who were blinded to patient clinical information. Any disagreements were resolved by discussion.
Statistical analysis
Statistical analysis was performed using SPSS (V.25.0, IBM). Continuous variables were described as mean±SD, whereas categorical variables were expressed as percentages. Independent sample t-tests were used to compare age, lesion maximum diameter and CT attenuation value between pneumonic-type PPL and pneumonia. A χ2 test was used to compare the frequencies of clinical information and CT image characteristics between pneumonic-type PPL and pneumonia. Moreover, multivariate logistic regression analysis with generalised estimating equation correction was performed to calculate the OR and the corresponding 95% CI of the independent predictors. Receiver operating characteristic curve analysis was performed for the model built by all independent predictors to differentiate pneumonic-type PPL and pneumonia. The diagnostic performance was assessed by the area under the curve (AUC), calibration curve and decision curve analysis (DCA). A P value of <0.05 was considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Results
Patient demographics
A total of 227 patients (mean age±SD, 58.4±12.9 years; 132 men and 95 women) with pneumonic-type PPL (n=72) and pneumonia (n=155) were enrolled in this study. Among 72 patients with pneumonic-type PPL, 50 (69.4%) cases had mucosa-associated lymphoid tissue (MALT) lymphoma, 18 (25.0%) cases had diffuse large B-cell lymphoma and 4 (5.6%) cases had Hodgkin lymphoma. The clinical data are summarised in table 1.
The clinical characteristics between pneumonic-type PPL and pneumonia
Clinical and paraclinical features between pneumonic-type PPL and pneumonia
As shown in table 1, the patients with pneumonia showed a higher prevalence of sputum (66.5% vs 44.4%, p=0.002) and fever (52.3% vs 9.7%, p<0.001) than those with pneumonic-type PPL. Leucocytosis (36.1% vs 19.4%, p=0.011) and elevation of C reactive protein level (69.0% vs 30.6%, p<0.001) were more commonly observed in patients with pneumonia than those with pneumonic-type PPL. No significant differences were found in other clinical information between the two groups (all p>0.05).
CT imaging features between pneumonic-type PPL and pneumonia
In pneumonic-type PPL, with air bronchogram was seen in 54 (75.0%) patients, bronchiectasis in 43 (59.7%) patients, a halo sign in 52 (72.2%) patients, pleural traction in 41 (56.9%) patients, pleural effusion in 19 (26.4%) patients and lymphadenopathy in 39 (54.2%) patients. The detailed CT features of the two groups are shown in table 2. Bronchiectasis (59.7% vs 28.4%, p<0.001) and halo sign (72.2% vs 36.1%, p<0.001) were more frequently present in pneumonic-type PPL than pneumonia (figure 1). The patients with pneumonia showed a higher prevalence of pleural effusion (60.0% vs 26.4%, p<0.001) than those with pneumonic-type PPL. The pneumonic-type PPL had a higher CT attenuation value (37.0±9.6 HU vs 32.5±5.0 HU, p<0.001) than pneumonia. However, no significant differences were observed in other CT characteristics between the two groups (all p>0.05).
Representative images of CT imaging characteristics. (A,B) CT images of the lung of a female patient in her mid-70s indicate the consolidation with bronchiectasis (long arrowhead) in the right upper lobe. (C) Pathology confirmed diffuse large B-cell lymphoma (H&E staining, × 200). (D,E) CT images of the lung of a male patient ages 55–60 years indicate the consolidation with air bronchogram (short arrowhead) in the right middle lobe. (F) Pathology confirmed pneumonia (H&E staining, × 200).
Comparison of CT imaging features between pneumonic-type PPL and pneumonia
Multivariate logistic regression analysis
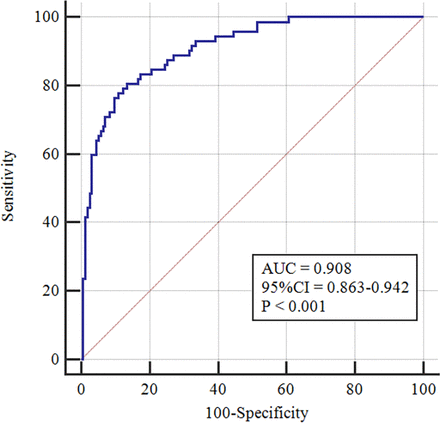
By multivariate logistic regression analysis, sputum (OR, 0.312; 95% CI, 0.134 to 0.724, p=0.007), fever (OR, 0.105; 95% CI, 0.037 to 0.297, p<0.001), elevation of C reactive protein level (OR, 0.411; 95% CI, 0.177 to 0.954, p=0.038), halo sign (OR, 7.515; 95% CI, 3.113 to 18.144, p<0.001), bronchiectasis (OR, 3.327; 95% CI, 1.440 to 7.687, p=0.005), pleural effusion (OR, 0.202; 95% CI, 0.088 to 0.465, p<0.001) and CT attenuation value (OR, 1.079; 95% CI, 1.016 to 1.145, p=0.013) were the independent predictors of pneumonic-type PPL (figure 2). The new model, constructed using independent predictors, showed great diagnostic performance in differentiating pneumonic-type PPL from pneumonia, with an AUC of 0.908 (95% CI, 0.863 to 0.942), an accuracy of 84.58% (95% CI, 79.88% to 89.28%), a sensitivity of 80.56% (95% CI, 74.67% to 86.16%) and a specificity of 86.45% (95% CI, 82.73% to 90.35%) (figure 3). The calibration curve (online supplemental figure 1) and DCA (online supplemental figure 2) showed that the model provided a good prediction ability for distinguishing pneumonic-type PPL from pneumonia.
The independent predictors of the presence of pneumonic-type primary pulmonary lymphoma were shown.
Receiver operating characteristic curve shows the diagnostic efficiency of the combined model by the independent predictors. AUC, area under the curve.
Discussion
PPL and pneumonia are two conditions that require different treatment approaches. Yet, distinguishing PPL from pneumonia may be challenging, because they both present as consolidation on CT imaging,12 13 which calls for accurate diagnosis. Our study compared the clinical and imaging features between two disease entities and developed a model using independent predictors. The model demonstrated excellent diagnostic performance with an AUC of 0.908.
In our study, pulmonary MALT lymphoma comprised 69.4% of all cases and was the most common lymphoma type, which was in accordance with the literature.14 15 In agreement with a previous study,3 we found that in pulmonary lymphoma that appeared as consolidation on CT imaging, the proportion of MALT lymphoma was relatively higher than in other lymphoma types. In our study, we showed that sputum, fever, leucocytosis (36.1% vs 19.4%, p=0.011) and the elevation of C reactive protein level (69.0% vs 30.6%, p<0.001) were more commonly observed in patients with pneumonia than those with pneumonic-type PPL (all p<0.05). Patients with pneumonic-type PPL usually remain asymptomatic due to the indolent nature of lymphoma.16 Also, previous studies consider anaemia a common clinical characteristic of lymphoma.17 However, no significant difference was observed in haemoglobin levels between pneumonic-type PPL and pneumonia in our study (p>0.05), which may be explained by different sample sizes.
We also found that the halo sign was more frequently (72.2% vs 36.1%, p<0.001) present in pneumonic-type PPL than in pneumonia (p<0.001), which was in line with previous research projects.3 18 Several studies consider the halo sign a significant characteristic of PPL.18 The halo sign of pneumonic-type PPL indicates the infiltration of the surrounding interstitium by sparsely arranged tumour cells.18 However, the halo sign of pneumonia may indicate inflammatory exudative oedema or alveolar haemorrhage surrounding consolidation.19 Therefore, the halo sign of pneumonia is less dense and more extensive than that of PPL. In our study, the halo sign in the PPL group was more frequent than in the pneumonia group, which could arise from longer establishment of tumorous infiltration for PPL than inflammation around the pneumonia.
In addition, pulmonary MALT lymphoma was the most common lymphoma (69.4%) in our study. Because tumour cells of pulmonary MALT lymphoma grow along the interstitial lung and bronchial submucosa, and there was no destruction of the bronchial wall,6 15 20 a large number of patients with pneumonic-type PPL (75.0%) had air bronchogram in our study, which is in line with a previous study.21 Similarly, the bronchial wall was not destroyed by inflammatory pneumonia changes.22 Therefore, an air bronchogram was also frequently seen in patients with pneumonia. In spite of the high frequency of air bronchograms in PPL patients, the difference in the present study was not significant (p>0.05). However, we found bronchiectasis was more frequently present in pneumonic-type PPL (59.7% vs 28.4%, p<0.001) than pneumonia (p<0.001). Consistent with our findings, Deng et al21 showed that bronchiectasis is strongly suggestive of PPL. The dilated airway may be caused by alveolar collapse or substantial destruction of the adjacent airway.21 In addition, PPL is poorly compressive to the surrounding tissues.
Kurtin et al showed that pleural invasion, as indicated by pleural effusion, is absent in PPL.23 Similar to their observations,23 we found that pleural effusions more often appear in pneumonia than pneumonic-type PPL (60.0% vs 26.4%, p<0.001). This is mainly due to the fact that pleural effusion is mostly the result of inflammatory changes that are more common in pneumonia.24
The present study showed that pneumonic-type PPL had a higher CT attenuation value than pneumonia (37.0±9.6 HU vs 32.5±5.0 HU, p<0.001). In some cases, the lymphomatous infiltrate became dense and fused, producing lung consolidation, interstitial sclerosis and replacement of the lung parenchyma.15 Therefore, this explains the higher CT attenuation value of pneumonic-type PPL.
Identifying these independent predictors may help making an initial diagnosis based on these features and proceed to experimental treatments. For example, when a patient presents with sputum, fever, elevated C reactive protein and pleural effusion, it is more suggestive of an infectious lesion and antibiotic management is recommended.
The present study has several limitations. First, this was a retrospective study, which may lead to bias. Second, non-enhanced CT images were collected in this study, and imaging features on contrast-enhanced CT were not studied in detail. Third, CT imaging was acquired from different scanners at different centres. However, their CT protocol was similar. In particular, tube voltage, a major parameter affecting CT value,25 was consistent. Fourth, detailed subtypes of lymphoma and pneumonia were not specifically studied. Fifth, although our study is a multicentre study, we have not performed validation of the model considering the sample size.
Conclusion
Our findings confirm that pneumonic-type PPL and pneumonia have different clinical and imaging features. The halo sign, bronchiectasis and higher CT attenuation value are suggestive of pneumonic-type PPL. Also, the sputum, fever, leucocytosis, C reactive protein level and pleural effusion usually were in favour of patients with pneumonia. These differential features between the two groups could be beneficial in guiding early diagnosis, evaluation and subsequent initiation of therapy.
Data availability statement
Data are available upon reasonable request. The original contributions presented in the study are included in the article/online supplemental material. Further inquiries can be directed to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
Institutional review board approval was obtained, and patient informed consent was waived due to the retrospective nature of this study.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
SL and LW contributed equally.
Contributors SL and NC initiated and designed the experiments. TQ-X and LW drafted the manuscript. SL analysed the data and interpreted the data. BX-J provided the relevant clinical information and valuable comments. SZ and XM-W revised the manuscript, obtained funding and supervised the study. All authors read and approved the final manuscript. XM-W is the guarantor for this work.
Funding The present study was supported by the National Natural Science Foundation of China (grant numbers 81871354 and 82271993) and Academic promotion programme of Shandong First Medical University (grant number 2019QL023).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.