Article Text
Abstract
Background SMARTTOUCH SURROUNDFLOW (STSF) catheter is the new generation of SMARTTOUCH (ST) catheter with an upgraded irrigation system for radiofrequency catheter ablation (RFCA) in patients with atrial fibrillation (AF).
Methods This systematic literature review searched the major English and Chinese bibliographic databases from 2016 to 2022 for any original clinical studies assessing the STSF catheter for RFCA in AF patients. Meta-analysis with a random effects model was used for evidence synthesis.
Results Pooled outcomes from 19 included studies indicated that STSF catheter was associated with a significantly shorter procedure time (weighted mean difference (WMD): −17.4 min, p<0.001), shorter ablation time (WMD: −6.6 min, p<0.001) and lower catheter irrigation fluid volume (WMD: −492.7 mL, p<0.001) than ST catheter. Pooled outcomes from four included studies with paroxysmal AF patients reported that using the STSF catheter for RFCA was associated with a significantly shorter ablation time (WMD: −5.7 min, p<0.001) and a lower risk of 1-year postablation arrhythmia recurrence (rate ratio: 0.504, p<0.001) than the SURROUNDFLOW (SF) catheter. Significant reductions in procedure time and ablation time associated with the STSF catheter were also reported in the other four studies using non-ST/SF catheters as the control. Overall complications of STSF catheter and control catheters were comparable.
Conclusions Using the STSF catheter was superior to using the ST catheter to conduct RFCA for AF by significantly reducing procedure time, ablation time, fluoroscopy time and irrigation fluid volume. The superiority of the STSF catheter over the SF catheter and other non-ST/SF catheters for RFCA needs further confirmation.
- cardiology
- systematic review
- cardiac surgery
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Improve the generalisability of the pooled evidence by updating the published evidence and including studies published in Chinese journals.
Conduct heterogeneity analyses, sensitivity analysis and publication bias analysis to confirm the robustness of the pooled evidence.
Most of the included studies in this review were observational studies that could introduce heterogeneity in the pooled evidence.
The pooled evidence is robust for the comparisons between SMARTTOUCH SURROUNDFLOW catheter and SMARTTOUCH catheter but not for the comparisons between the other catheter types due to paucity of existing evidence.
Introduction
Radiofrequency catheter ablation (RFCA) plays a critical role in managing atrial fibrillation (AF), which affects 1.6% of the Chinese adult population and is rising in prevalence along with the ageing population in China.1 RFCA was originally conducted using a non-contact force (CF)-sensing catheter, whose use is now discouraged due to the inadequate lesion formation caused by insufficient CF or complications (such as cardiac perforation and atrioesophageal fistula) caused by excessive CF.2 Thus, a CF-sensing catheter was developed to improve ablation outcomes and safety. The THERMOCOOL SMARTTOUCH (ST) catheter is one of the CF-sensing catheters widely used for RFCA. The ST catheter is equipped with a technology that can measure the CF generated by the catheter tip on the myocardium and an irrigation system that cools the tip of the electrode catheter during ablation and allows high radiofrequency energy ablation without overheating at the electrode-tissue interface.3 To enhance the cooling effects on the tip of the catheter electrode, surround flow technology was developed by equipping the catheter porous tip with 56 tiny holes, which make conduits for optimal fluid pressure distribution in the catheter tip. As the new generation of a catheter with advanced irrigation technology, the STSF catheter combines both CF and SF technologies to optimise ablation outcomes, protect cardiac function and reduce the risk of developing eschar during ablation.4 According to a meta-analysis of four clinical trials published before 2020, the STSF catheter was superior to the ST catheter in procedure outcomes by reducing the procedure time, fluoroscopy time and catheter irrigation infusion volume.5 However, this meta-analysis was unable to assess the robustness of the pooled evidence due to the small number of included studies. Additionally, this review did not perform any analysis to address the heterogeneity and publication bias in the pooled evidence. With accumulated evidence from recently published studies assessing STSF catheter ablation in patients with AF, we conducted this systematic literature review (SLR) aiming to add more evidence from multiple sources (journals published in Chinese and recent conference proceedings) and including studies comparing STSF versus catheters other than ST to better comprehend the values of STSF catheter for RFCA in AF patients. Thus, this SLR could be a timely evidence source to support the management of AF with catheter ablation in the countries where STSF was considered a new technology to improve ablation outcomes in AF patients.
Materials and methods
This study was designed as an SLR using major English-language and Chinese-language bibliographic databases to identify published, peer-reviewed clinical studies comparing the STSF catheter against other ablation catheters for procedural characteristics and clinical outcomes associated with RFCA in AF patients. This SLR was reported by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 Statement.6
Study eligibility criteria
This SLR set both inclusion and exclusion criteria to identify randomised clinical trials or observational studies (retrospective or prospective cohort studies) comparing the STSF catheter with other ablation catheters for AF. The study inclusion criteria are as follows: (1) including AF patients who underwent RFCA; (2) assessing STSF against any other type of ablation catheter for RFCA in adult patients with AF; (3) reporting procedural characteristics and clinical outcomes associated with ablation catheter during and/after RFCA in AF patients and (4) designed as a clinical trial or observational study. The exclusion criteria of this SLR are as follows: (1) preclinical (in vivo or in vitro) studies, case studies, case reports, non-original research articles (eg, correspondence, editorials, commentaries, overviews, summaries, communications, consensus guidelines) and reviews; (2) any cohort that includes patients with ablation for arrhythmias other than AF; (3) single-arm studies assessing STSF without control and (4) inadequate information.
Information sources and search strategies
Given that RFCA has been implemented for AF treatment for over 20 years in China, many clinical studies assessing various ablation catheters for AF have been published in Chinese clinical journals. Therefore, this SLR explored major English bibliographic databases (MEDLINE, Embase, Web of Science and the Cochrane Library) and three major Chinese bibliographic databases (WANFANG, VIP and China National Knowledge Infrastructure) as the data sources. To align with the time of STSF approval in 2016, the literature search period was set from 1 January 2016, to the date when the literature search was first conducted (31 July 2022). Grey literature search was conducted by searching the proceedings of the Heart Rhythm Society annual conference, the Society for Cardiovascular Angiography and Interventions annual conference, the European Heart Rhythm Association annual conference and the Asia Pacific Heart Rhythm Society annual conference in 2021 and 2022 for any relevant but not fully published studies. The trial registry databases, including ClinicalTrials.gov, European Union Clinical Trials Register and International Clinical Trials Registry Platform, were searched as well for any missing studies. To ensure that all relevant evidence is captured, this study only combined the keywords for AF and STSF to develop the search strategy for each bibliographic database and grey literature search. Search strategies is shown in online supplemental table 1.
Supplemental material
Literature selection process
Two reviewers conducted the literature selection independently after which the search hits were pooled. Then, they deleted duplicate results and identified additional studies from the left references for further eligibility assessment, which included the exclusion of irrelevant references and retrieving full publications of the relevant references. The source references reporting relevant outcome information from clinical guidelines, literature review and health economic research were cross checked with the identified references to avoid missing studies. The developed inclusion and exclusion criteria were used to determine the study eligibility after a full publication review. The exclusion reasons during the literature selection process were documented for records. Any disagreement on study eligibility between the two reviewers was resolved by consulting with the study lead.
Data collection process
Excel-based data extraction forms were developed specifically to guide the data collection from the full publications of included studies. The designed data extraction form was tested using one included study to align with definitions of the planned data items for extraction. Two reviewers were fully trained on how to use the data extraction forms and the definitions of data items. The two reviewers conducted data extraction independently. The extracted information from the two reviewers was further cross-checked by the third reviewer, which corrected any inconsistent information by verifying the information source. The study lead reviewed all extracted information for any abnormal information before evidence synthesis.
Data items
The full publication of the included studies was reviewed to collect the following information: (1) study characteristics such as country setting, study design, and patient inclusion and exclusion criteria; (2) study arm information including the arm definition, sample size and patient baseline characteristics (demographics, AF-related clinical characteristics and comorbidities); (3) ablation catheter type; (4) outcome measures that included procedural characteristics (procedure time, ablation time, fluoroscopy time, irrigation fluid volume), clinical outcomes (acute procedural success of pulmonary vein isolation (PVI), 1-year postablation cardiac arrhythmia recurrence, ablation-related complications) and other relevant outcomes (eschar, use of diuretics and use of urinary catheter). Most of the included studies did not provide adequate information for the definitions of outcome measures except catheter irrigation fluid volume, fluoroscopy time and acute procedural success of PVI.
Study risk of bias assessment
This SLR used Newcastle-Ottawa Scale (NOS)7 to assess the study quality of the included studies. Based on the recommendation from previous research,8 this SLR classified included studies as good quality (NOS 8–9), fair quality (NOS 5–7) and poor quality (NOS 0–4). This SLR included one randomised clinical trial, which was published as a conference abstract and did not provide adequate information for the quality assessment using the Jadad score.9 Two reviewers used NOS to assess the fully published studies independently. Any disagreement on assessment was discussed with the study lead to reach a consensus.
Effect measures
This SLR extracted any reported effect measures from the included studies. The extracted effect measures were standardised according to their original definitions in the included studies and the selected effect measures for evidence synthesis included procedural characteristics and clinical outcomes. This SLR used weighted mean difference (WMD) to present the pooled procedural characteristics for the comparisons of procedure time, ablation time, fluoroscopy time and catheter irrigation fluid volume. The pooled clinical outcomes for the comparisons of acute procedural success of PVI, 1-year postablation arrhythmia recurrence and RFCA-related overall complications were presented with a rate ratio (RR).
Synthesis methods
The extracted data were standardised and categorised by AF types (paroxysmal AF, persistent AF and unspecified AF); control catheter types (ST, SF, CELSIUS catheter, DiamondTemp and NAVISTAR); patient characteristics (age, gender distribution, AF type distribution, disease duration after the diagnosis of AF, left ventricular ejection fraction (LVEF), left atrium diameter, CHA2DS2 VASc and comorbidities); and effect measures for RFCA procedural characteristics and clinical outcomes. The reported outcomes from the included studies comparing STSF versus the same control catheter were first pooled for evidence synthesis using a pairwise meta-analysis method, which used a random-effect model to consider the variance between the included studies and within each included study. Heterogeneity in the conducted meta-analysis was assessed using the I2 method. The included studies were stratified by AF type for subgroup analysis if the heterogeneity in the pooled outcomes was significant. Further exploration of potential heterogeneity sources was conducted by excluding the studies reporting different patient characteristics if significant heterogeneity was still detected in the pooled outcomes from the subgroup analysis. The leave-one-out sensitivity analysis was conducted to determine the robustness of the overall pooled outcomes for the meta-analysis including three or more eligible results. The Egger’s test was also performed to assess publication bias for overall pooled outcomes from 10 or more eligible results. This SLR used the statistical software R to conduct the described analyses. Original results from included studies were reported when the meta-analysis was not feasible.
Results
Study selection
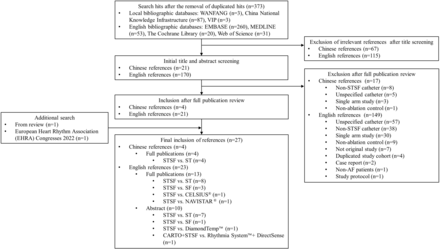
This study initially identified 373 unique references from the search of the included English and Chinese bibliographic databases. One-hundred and eighty-two were excluded due to irrelevance following the review of the titles and abstracts of the initial batch of papers. Following the study eligibility assessment of the full publications of the remaining 191 papers, 25 met the inclusion criteria. The search of conference proceedings and review articles identified two additional eligible studies. Thus, a total of 27 studies are included in our SLR. The flow chart of the study identification process is illustrated in figure 1.
Literature search flow chart for identifying eligible studies. AF, Atrial fibrillation; SF, SURROUNDFLOW; ST, THERMOCOOL SMARTTOUCH; STSF, SMARTTOUCH SURROUNDFLOW.
Characteristics and qualities of included studies
The included 27 studies assessed the procedural characteristics and clinical outcomes associated with STSF relative to ST (in 19 studies), SF (in 4 studies) and other four non-STSF/SF catheters (1 study for each non-STSF/SF catheter), respectively. This SLR only included one randomised clinical trial and the rest of the included studies were observational studies, including 13 retrospective studies and 13 prospective studies. This SLR included four studies published in Chinese. The studies published in English included 3 studies from the USA, 13 studies from Europe and 7 studies from other regions. Among the included studies, 17 studies were fully published and 10 studies were published in conference proceedings. Even though all these studies included patients who underwent RFCA for AF, 7 studies solely included patients with paroxysmal AF, 1 study only included patients with persistent AF and 19 studies included patients with either paroxysmal or persistent AF. According to the reported patient baseline characteristics in these included studies, the study patients were characterised with relatively old age (mean age range: 58.0–67.5 years), high CHA2DS2 VASc score (mean range: 1.3–2.7) and prevalent cardiovascular comorbidities, which included hypertension (30.4%–98.0%), coronary heart disease (8.3%–29.2%) and heart failure (17.8%–41.7%). Of the 17 studies assessed for study quality, 7 studies had good quality and 10 studies had fair quality. The study characteristics and main extracted information from these included 27 studies are summarised in online supplemental table 2.
Synthesised evidence from the included studies comparing the STSF catheter with the ST catheter
Of the included 19 studies comparing STSF with ST, 13 studies10–22 included patients with unspecified AF (persistent or paroxysmal AF) and 6 studies23–28 included patients with paroxysmal AF. The synthesised outcomes included procedural characteristics (procedure time, ablation time, fluoroscopy time and irrigation fluid volume), primary clinical outcomes (acute procedural success of PVI, 1-year postablation arrhythmia recurrence and overall complications), and other ablation-related clinical outcomes that included foley catheter use, diuretics use and eschar development.
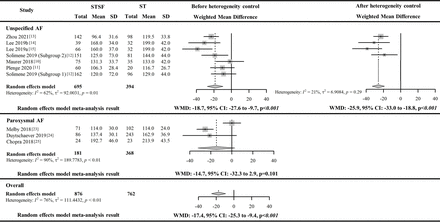
Procedural characteristics: procedure time
Overall, 9 included studies with 10 eligible results10–15 23–25 report RFCA procedure time (876 operated with STSF and 762 operated with ST). The overall pooled outcomes from nine included studies showed that STSF was associated with significantly shorter procedure time than ST (WMD −17.4 min, 95% CI −25.3 to −9.4 min, p<0.01); however, this pooled outcome has considerable heterogeneity (I2=76%, p<0.01). The pooled outcomes from the stratified studies by AF types identified significantly shorter procedure time associated with the STSF catheter from the studies with unspecified AF patients (WMD −18.7 min, 95% CI −27.6 to −9.7 min, p<0.001) but not from the studies with paroxysmal AF patients (WMD −14.7 min, 95% CI −32.3 to 2.9 min, p=0.101). Because the heterogeneity of the pooled evidence from the six studies with unspecified AF patients was still significant, we reviewed these six studies to further explore the potential heterogeneity sources.
We found that two studies10 11 and a subgroup within one study12 included patients who were likely to be different from those in other studies in AF duration, left atrial diameter/volume, the proportion of patients with paroxysmal AF and proportion of patients with cardiomyopathy. After excluding the results from these four studies in the meta-analysis, the shorter procedure time of the STSF catheter remained statistically significant (WMD −25.9 min, 95% CI −33.0 to −18.8 min, p<0.001) with non-significant heterogeneity (I2=21%, p=0.29), suggesting that these characteristics are potential heterogeneity sources.
The leave-one-out sensitivity analysis indicated that the point estimation of the overall pooled difference in procedure time between the STSF catheter and the ST catheter had a relatively narrow range (from −15.2 min to −19.9 min). In addition, Egger’s test did not detect significant publication bias for the reported difference in procedure time between the STSF catheter and the ST catheter from the included nine studies (p=0.768). The pooled difference in the procedure time between the STSF catheter and the ST catheter is illustrated in figure 2. The other reported outcomes are listed in online supplemental figure 1 and online supplemental figure 2.
Forest plot for the paired meta-analysis of the included studies for the difference in RFCA procedure time (minutes) between STSF catheter and ST catheter. AF, Atrial fibrillation; RFCA, radiofrequency catheter ablation; STSF, SMARTTOUCH SURROUNDFLOW; ST, THERMOCOOL SMARTTOUCH; WMD, weighted mean difference.
Procedural characteristics: ablation time
Twelve included studies10–17 23–26 with 13 eligible results reported the ablation time associated with using STSF and ST to conduct RFCA in 1870 patients with AF (992 operated with STSF and 878 with ST). The pooled differences in the ablation time of the two catheters favoured the STSF catheter (WMD: −6.6 min, 95% CI −12.5 to −0.6 min, p=0.031) with significant heterogeneity (I2=98%, p<0.01). To control the potential heterogeneity associated with AF type, this SLR performed a subgroup meta-analysis for this outcome by including the stratified studies by the AF types of study patients (paroxysmal AF vs unspecified AF). The pooled difference in ablation time between the two catheters remained significant in the meta-analysis of the studies with unspecified AF patients (WMD −8.6 min, 95% CI −16.9 to −0.4 min, p=0.039) but was not for the studies with paroxysmal AF patients (WMD −1.1 min, 95% CI −4.8 to 2.6 min, p=0.555). However, heterogeneity in the subgroup meta-analysis of the studies with unspecified AF patients was still significant (I2=98%, p<0.01) and brought our attention to further explore the potential heterogeneity sources in these studies. By reviewing the reported patient baseline characteristics from these included studies, we found four studies10–12 16 with obviously different patient characteristics (AF duration, left atrial diameter/volume, the proportion of paroxysmal AF, proportion of patients with myopathy, Ablation Index value, baseline CHA2DS2 VASc score, saline flow rate) from the other studies. After excluding these four studies from the subgroup meta-analysis, the pooled difference in ablation time still favoured the STSF catheter with statistical significance (WMD −22.5 min, 95% CI −24.3 to −20.6 min, p<0.001) and low-level of heterogeneity (I2=0%, p=0.69), suggesting that these characteristics are potential heterogeneity sources.
The overall pooled difference in ablation time between the two catheters from the leave-one-out sensitivity analysis ranged from −7.5 min to −5.1 min. No significant publication bias was detected from the included 12 studies comparing the two catheters for ablation time during RFCA (Egger’s test: p=0.450). The pooled difference in the ablation time between the STSF catheter and the ST catheter is illustrated in figure 3. The other reported outcomes are listed in online supplemental figures 3 and 4 .
Forest plot for the paired meta-analysis of the included studies for the difference in ablation time (minutes) between STSF catheter and ST catheter. AF, atrial fibrillation; STSF, SMARTTOUCH SURROUNDFLOW; ST, THERMOCOOL SMARTTOUCH; WMD, weighted mean difference.
Procedural characteristics: irrigation fluid volume
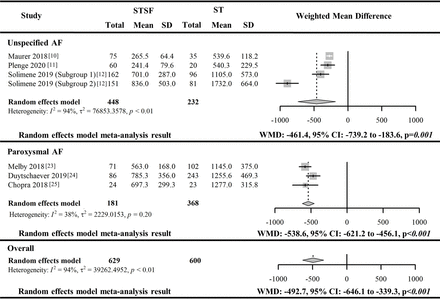
Six included studies10–12 23–25 with 1229 AF patients (629 operated with STSF and 600 with ST) reported catheter irrigation fluid volume during RFCA. The meta-analysis of the reported irrigation fluid volume associated with the two catheters from the six studies indicated a significantly lower irrigation volume for using STSF to conduct RFCA (WMD: −492.7 mL, 95% CI −646.1 to −339.3 mL, p<0.001). However, this pooled outcome was associated with significant heterogeneity (I2=94%, p<0.01). These six included studies were stratified by patient AF type (paroxysmal AF vs unspecified AF) to conduct a meta-analysis for the control of potential heterogeneity associated with AF types. The pairwise meta-analysis of the three studies with paroxysmal AF patients23–25 confirmed the significant reduction of catheter irrigation fluid volume (WMD: −538.6 mL, 95% CI −621.2 to −456.1 mL, p<0.001) with moderate but non-significant heterogeneity (I2=38%, p=0.20) for RFCA conducted by STSF catheter. However, significant heterogeneity (I2=94%, p<0.01) was found for the pooled difference in catheter irrigation fluid volume (WMD: −461.4 mL, 95% CI −739.2 to −183.6 mL, p=0.001) between the two catheters from the left three studies with unspecified AF patients.10–12 No further exploration of heterogeneity resources for this pooled outcome due to a limited number of studies reporting this outcome measure. The overall pooled difference in catheter irrigation fluid volume between the two catheters from the leave-one-out sensitivity analysis ranged from −532.1 mL to −427.3 mL.
The pooled difference in the catheter irrigation fluid volume between the STSF catheter and the ST catheter is illustrated in figure 4. The other reported outcomes are listed in online supplemental figure 5.
Forest plot for the paired meta-analysis of the included studies for the difference in catheter irrigation fluid volume (mL) between STSF catheter and ST catheter for RFCA. AF, atrial fibrillation; STSF, SMARTTOUCH SURROUNDFLOW; ST, THERMOCOOL SMARTTOUCH; WMD, weighted mean difference.
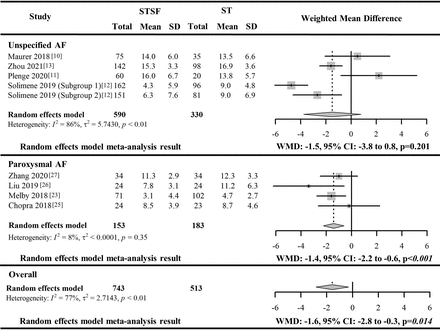
Procedural characteristics: fluoroscopy time
Eight included studies10–13 23 25–27 compared fluoroscopy time between STSF catheter and ST catheter used to conduct RFCA (four studies10–13 with unspecified AF patients and four studies23 25–27 with paroxysmal AF). The overall pooled difference in fluoroscopy time during RFCA between the two catheters showed that the STSF catheter was associated with significantly shorter fluoroscopy time than the ST catheter (WMD: −1.6 min, 95% CI −2.8 to −0.3 min, p=0.014); however, this pooled outcome was associated with significant heterogeneity (I2=77%, p<0.014). The included studies were further stratified by the patient AF types (paroxysmal AF vs unspecified AF) to conduct subgroup meta-analysis to explore potential heterogeneity associated with AF types. The subgroup meta-analysis including studies with paroxysmal AF patients confirmed the significantly shorter fluoroscopy time during RFCA conducted by STSF catheter (WMD −1.4 min, 95% CI −2.2 to −0.6 min, p<0.001) with a low level of heterogeneity (I2=8%, p=0.35).23 25–27 However, the pooled difference in fluoroscopy time between the two catheters from the subgroup meta-analysis of five eligible results from the four studies with unspecified AF patients10–13 did not reach statistical significance and also had substantial heterogeneity. No further exploration of heterogeneity sources for this subgroup meta-analysis due to a limited number of included studies reporting this outcome. The overall pooled difference in fluoroscopy time between the two catheters from all included studies in the leave-one-out sensitivity analysis ranged from −1.9 min to −1.4 min.
The results of the meta-analysis of the included eight studies reporting fluoroscopy time associated with STSF catheter and ST catheter are illustrated in figure 5. The other reported outcomes are listed in online supplemental figure 6.
Forest plot for the paired meta-analysis of the included studies for the difference in fluoroscopy time between STSF catheter and ST catheter for RFCA. AF, atrial fibrillation; STSF, SMARTTOUCH SURROUNDFLOW; ST, THERMOCOOL SMARTTOUCH; WMD, weighted mean difference.
Primary clinical outcomes
Thirteen studies10–17 22–24 26 28 reported primary clinical outcomes, including the acute procedural success of PVI, 1-year postablation cardiac arrhythmia recurrence and overall complications related to RFCA. The overall pooled RR for acute procedure success,10 12 14–17 26 28 1-year postablation cardiac arrhythmia recurrence,10 13 17 22 28 and overall complications11 14 16 17 23 24 26 28 from these studies were 0.995 (95% CI 0.976 to 1.014, p=0.592), 0.727 (95% CI 0.355 to 1.490, p=0.384) and 0.766 (95% CI 0.299 to 1.959, p=0.578), respectively, without reaching statistical significance. Among these three pooled outcomes, only the pooled RR for 1-year postablation arrhythmia recurrence between the two catheters was associated with significant heterogeneity (I2=68%, p<0.01). Subgroup meta-analysis including stratified studies by patient AF types (paroxysmal AF vs unspecified AF) was unable to homogenise the pooled RR for 1-year postablation cardiac arrhythmia recurrence between the two catheters. The leave-one-out sensitivity analyses for the three pooled outcomes observed a narrow range for pooled RR for the acute procedural success of PVI (95% CI 0.993 to 0.999) but wide ranges for 1-year postablation cardiac arrhythmia recurrence (95% CI 0.555 to 0.929) and overall complications (95% CI 0.600 to 0.927). All reported outcomes are illustrated in online supplemental figures 7–10.
Other ablation-related clinical outcomes
Three included studies reported other ablation-related clinical outcomes. Two studies23 24 (502 paroxysmal AF patients) reported significantly lower utilisations of the foley catheter (RR 0.506, 95% CI 0.393 to 0.651, p<0.001) without heterogeneity (I2=0%, p=0.68). One study25 with 47 paroxysmal AF patients reported STSF catheter was associated with a significantly lower risk of diuretics use (RR 0.050, 95% CI 0.003 to 0.819, p=0.036). In addition, one study27 with 68 paroxysmal AF patients reported that STSF catheter was associated with a reduced risk of eschar formation during ablation without reaching statistical significance (RR 0.143, 95% CI 0.008 to 2.663, p=0.192). The pooled outcomes are illustrated in online supplemental figure 11.
Synthesised evidence from the studies comparing the STSF catheter with the SF catheter
This SLR identified four studies29–32 comparing STSF with SF for procedural characteristics and clinical outcomes in AF patients. One study29 with a small sample size (26 using STSF catheter and 26 using SF catheter) reported significantly longer RFCA procedure time (mean difference: 20.0 min, 95% CI 2.9 to 37.1 min, p=0.022) and fluoroscopy time (mean difference: 4.0 min, 95% CI 1.1 to 6.9 min, p=0.007) in the STSF group. The meta-analysis including 2 studies29 30 with 252 patients did not identify significant differences in both acute procedure success of PVI and ablation-related complications between the two catheters. One study31 with 395 patients with paroxysmal AF (298 using STSF and 97 using SF) reported significantly shorter ablation time (mean difference: −5.7 min, 95% CI −8.4 to −3.1 min, p<0.001). The pooled RR for 1-year postablation arrhythmia recurrence between the two catheters from the two studies31 32 favoured the STSF catheter with statistical significance (RR 0.503, 95% CI 0.379 to 0.667, p<0.001, heterogeneity test: I2=0%, p=0.98) when compared with SF catheter. The reported RFCA-related outcomes from the four studies are summarised in table 1. The pooled outcomes are illustrated in online supplemental figures 12–15.
Summary of the pooled differences in RFCA-related outcomes between STSF catheter and SF catheter in AF patients
Reported outcomes between STSF catheter and non-ST/SF catheter
This SLR identified four studies comparing STSF with four non-ST/SF catheters which were the CELSIUS catheter,33 DiamondTemp catheter,34 DirectSense catheter guided by Rhythmia System35 and NAVISTAR catheter.36 The four studies reported that the STSF catheter was associated with significantly shorter RFCA procedure time than the DiamondTemp catheter(mean difference −20.6 min, 95% CI −32.5 to −8.7 min, p<0.001) and NAVISTAR catheter (mean difference −30.0, 95% CI −39.9 to −20.1 min, p<0.001); significantly shorter ablation time than NAVISTAR catheter (mean difference −15.0 min, 95% CI −20.5 to −9.5 min, p<0.001); and significantly shorter fluoroscopy time than DirectSense catheter guided by Rhythmia System (mean difference −7.0 min, 95% CI −10.9 to −3.1 min, p<0.001) and NAVISTAR catheter (mean difference −2.0 min, 95% CI −2.8 to −1.2 min, p<0.001). However, 1 study with 116 patients with persistent or paroxysmal AF34 reported that the STSF catheter was associated with a significantly longer ablation time than the DiamondTemp catheter (mean difference: 4.1 min, 95% CI 2.0 to 6.2 min, p<0.001). None of these four studies reported any significant differences in the rates of ablation-related overall complications between the STSF catheter and the four non-ST/SF catheters.
Discussion
Compared with a similar SLR published in 2020,5 our SLR was designed with an expansive search period and search scope which has resulted in the inclusion of a larger pool of studies and much more robust evidence to demonstrate the values of STSF catheter for RFCA in AF patients. For example, our SLR captured and studied significantly more studies than the aforementioned SLR (27 studies vs 4 studies). Additionally, not only did our SLR include studies comparing STSF with ST but also with SF and other ablation catheters in AF patients; in contrast, the other SLR only included studies comparing STSF with ST. Furthermore, our SLR synthesised evidence for more outcomes than the previous SLR and conducted additional heterogeneity analysis and publication bias assessment to make the pooled findings more robust. Therefore, our SLR should be more informative regarding the clinical values of STSF for RFCA in AF patients.
According to the studies reviewed in this SLR, the STSF catheter was mainly studied in comparison with the ST catheter in AF patients. As the STSF catheter evolved from the ST catheter by upgrading the irrigation system to improve procedural characteristics, the STSF catheter contains all the features of the ST catheter such as the CF technology and advanced irrigation system that provides uniform cooling at half the flow rate of ST catheter and facilitates the process of fluid management.4 The pooled evidence for the outcomes that were compared between the two catheters in our SLR aligned with the expected impact of the advanced irrigation system of STSF. For example, the pooled evidence showed that the STSF catheter significantly save RFCA procedure time (17.4 min, p<0.001), ablation time (6.6 min, p=0.031) and fluoroscopy time (1.6 min, p=0.016) with significantly reduced catheter irrigation fluid volume (492.7 mL, p<0.001) relative to ST catheter. These benefits could potentially improve the performance efficiency of RFCA and enhance the capacity of conducting RFCA in hospital settings. The substantial reduction in the irrigation volume of STSF could substantially limit the cardiac burden due to catheter irrigation infusion and make ablation treatment safer to treat AF with heart failure. Even though the pooled outcome for reduced fluoroscopy time was statistically significant, the estimated reduction of fluoroscopy time by STSF in this review was unlikely to be substantial and this finding should be interpreted with caution. As a new technology, STSF could be often used with more fluoroscopy to confirm the position of catheter during the learning process. With more use of STSF in real-world settings, the benefits of STSF in reducing occupational health hazards during RFCA could be better demonstrated in future studies.
The pooled evidence also indicates that primary clinical outcomes, including acute procedure success of PVI, 1-year postablation arrhythmia recurrence and overall complications, are comparable for the STSF catheter and ST catheter. A possible explanation is that both catheters use the same CF technology, which is the primary driver of the ablation effects.37 However, the advanced irrigation system of the STSF could bring more clinical benefits to AF patients with heart failure. According to the reported patient characteristics from the included studies, AF patients are characterised by old age (mean age range: 58.0–67.5 years old) and a high prevalence of heart failure (17.8%–41.7%). The fluid infusion through the catheter during RFCA could stress the heart and deteriorate the cardiac function in patients with heart failure. Even though RFCA has been proven to improve cardiac function (indicated by LVEF38), previous studies observed a high rate of developing acute heart failure (4.9%–26.1%) after open-irrigated catheter ablation39–41; the development of acute heart failure after ablation in these studies was likely due to excessive infusion fluid during ablation procedure as patients with developed acute heart failure after ablation was associated with significantly higher net fluid infusion volume during ablation than those without developing acute heart failure. Thus, the substantial reduction of the catheter irrigation infusion volume of the STSF catheter could lower the burden of RFCA on the cardiac load and potentially reduce the risk of acute heart failure after RFCA.42 In addition, the shortened ablation time through STSF could make RFCA more tolerable for AF patients with heart failure who are prone to developing respiratory distress with the flat position required by the ablation procedure.43 Since AF patients are often complicated with heart failure due to old age and other cardiovascular conditions, future research should be encouraged to confirm the cardiac function-related benefits of STSF and generate robust evidence to inform clinical practices and guidelines regarding the appropriate applications of STSF catheter ablation for AF. Another potential clinical benefit of the improved irrigation system of STSF is the reduction of the risk of eschar due to the amplified cooling effects. Eschar occurs more often with unipolar radiofrequency ablation that generates excessive local temperature leading to the formation of eschar on the tissue surface; carbonisation; and thromboembolic complications; and even damage to the oesophagus and atrium, which induces serious complications such as atrial oesophageal fistula, atrial rupture and pulmonary vein stenosis.44 Because the STSF catheter has a more advanced irrigation system than the ST catheter, it is expected that the STSF catheter could be associated with a lower risk of eschar formation than the ST catheter. However, this SLT did not identify robust evidence to support this clinical benefit of STSF as only one study with a small sample size reported a non-significant trend for the reduced risk of eschar for STSF catheter.27
This SLR also identified four eligible studies comparing the STSF catheter with SF catheter and other four studies comparing the STSF catheter with non-ST/SF catheters. The pooled evidence from two eligible studies identified significantly reduced 1-year postablation arrhythmia recurrence for STSF catheter relative to SF catheter. Because these SF catheters were equipped with a similar irrigation technology as the STSF catheter but without CF technology, which mainly drives the ablation outcomes.37 The reported outcomes from the four studies comparing the STSF catheter with contemporary non-ST/SF catheters suggested that the STSF catheter could be better than the non-ST/SF catheter regarding the procedure characteristics, which included procedural time, ablation time and fluoroscopy time. However, these findings are not robust due to a limited number of studies (only one study comparing STSF with each non-ST/SF catheter) and the small sample size in each included study.
The generated evidence from this SLR should be interpreted with caution as most of the included studies were observational studies (26 observational studies and 1 randomised clinical trial) and the reported outcomes from the included studies were not pooled separately by study design. Thus, the pooled evidence in our review is likely to have the common limitations of observational studies that include bias, measurement bias and unknown confounders. These limitations could introduce heterogeneity in the pooled evidence in our review. Additionally, the included studies with small sample size could further introduce heterogeneity. That might explain why most of the overall pooled outcomes in this SLR had significant heterogeneity. This SLR did recognise that AF type could an important heterogeneity source as the persistent AF usually requires additional substrate ablation beyond PVI than paroxysmal AF. Thus, this SLR stratified the included studies by patient AF types to control heterogeneity in the pooled outcomes. This strategy seems to work well in reducing heterogeneity in the pooled outcomes from the studies only including paroxysmal AF patients. Due to insufficient studies, this SLR only tried to explore heterogeneity resources for procedure time and ablation time by further excluding studies with obviously different patient characteristics rather than conducting meta-regression analyses. The lack of definitions for some outcome measures in the included studies could introduce measurement bias and further increase the heterogeneity in the pooled evidence. In addition, this SLR does not have enough studies to explore the heterogeneity sources in other pooled outcomes. For the same reason, this SLR only assessed the publication bias for RFCA procedure time and ablation time. Given the fact that most of the included studies compared the STSF catheter with the ST catheter, the pooled evidence regarding the comparisons between STSF with non-ST catheters was not robust enough. Thus, this SLR did not grade the pooled evidence because of the limitations discussed above. Future research with adequate quality is still needed to confirm the generated evidence from this SLR and further explore the potential clinical benefits of using the STSF catheter to conduct RFCA for AF (such as preventing eschar and acute heart failure).
In summary, this SLR demonstrated that STSF is superior to ST catheter by reducing procedure time, ablation time, fluoroscopy time and irrigation fluid volume. Because both catheters use CF technology which is a key factor in determining ablation outcomes, it is not a surprise to see highly comparable acute procedure success of PVI and 1-year postablation arrhythmia recurrence between STSF catheter and ST catheter from the pooled evidence. Due to the lack of sufficient and robust evidence to support other clinical benefits of the STSF catheter relative to other catheters, such as preventing eschar and acute heart failure, more future studies with appropriate study designs and sufficient sample size are needed in this field.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
JL and GZ are joint first authors.
XL, SH, HL and SL are joint senior authors.
JL and GZ contributed equally.
XL, SH, HL and SL contributed equally.
Contributors JL, GZ, YW and XH formulated the research idea. JL, GZ, XL, SH, YW, XH, LT and WC developed the study protocol. JL, GZ, XL, SH, HL, SL and LT conducted the literature search, study quality assessment, data extraction and evidence synthesis. JL, GZ, XL, SH, XH, YW and WC drafted the manuscript based on the study findings. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. XH and YW are joint last authors.
XH is the guarantor of this manusript.
Funding This research was funded by Johnson and Johnson Medical (Shanghai) (no grant number).
Competing interests LT and WC are employed by contract research organisations that receive industry funds to conduct health economics and outcomes research. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.