Article Text
Abstract
Introduction Ischaemia/reperfusion injuries (IRIs) are associated with poorer survival of kidney grafts from expanded criteria donors. Preclinical studies have shown that mineralocorticoid receptor antagonists (MRAs) prevent acute and chronic post-ischaemic renal dysfunction by limiting IRI. However, data concerning the safety of MRAs in brain-dead donor patients are scarce. We seek to investigate the tolerance of MRAs on the haemodynamics in this population.
Methods and analysis CANREO-PMO is a randomised, controlled, single-centre, double-blind study. Brain-dead organ donors hospitalised in intensive care are randomised 1:1 after consent to receive 200 mg potassium canrenoate or its matching placebo every 6 hours until organ procurement. The primary outcome is a hierarchical composite endpoint that includes: (1) cardiocirculatory arrest, (2) the impossibility of kidney procurement, (3) the average hourly dose of norepinephrine/epinephrine between randomisation and departure to the operating room, and (4) the average hourly volume of crystalloids and/or colloids received. Thirty-six patients will be included. The secondary endpoints evaluated among the graft recipients are the: (1) vital status of the kidney graft recipients and serum creatinine level with estimated glomerular filtration rate (GFR) according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) at 3 months after renal transplantation, (2) percentage of patients dependent on dialysis and/or with an estimated GFR <20 mL/min/1.73 m2 at 3 months, (3) vital status of the kidney graft recipients at 3 months, and (4) vital status of the kidney graft recipients and creatinine levels (in μmol/L), with the estimated GFR according to CKD-EPI (in mL/min/1.73 m2), at 1 year, 3 years and 10 years after transplantation.
Ethics and dissemination This trial has full ethical approval (Comité de Protection des Personnes: CPP Ouest II-ANGERS, France), and the written consent of relatives will be obtained. Results will be reported at conferences, peer-reviewed publications and using social media channels.
Trial registration number NCT04714710.
- Renal transplantation
- INTENSIVE & CRITICAL CARE
- Clinical trials
- Transplant medicine
- End stage renal failure
- NEPHROLOGY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Renal transplantation
- INTENSIVE & CRITICAL CARE
- Clinical trials
- Transplant medicine
- End stage renal failure
- NEPHROLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first trial on mineralocorticoid receptor antagonist (MRA) tolerance in brain-dead donor patients.
A hierarchical composite endpoint to assess the overall tolerability of MRAs is used.
This phase II clinical trial is the prerequisite for an efficacy trial assessing MRA use in brain-dead donor patients to prevent ischaemia/reperfusion injuries in renal grafts.
This is a single-centre trial with a limited sample size.
This protocol does not evaluate the effects of potassium canrenoate on organs other than the kidney.
Introduction
The kidney is the most frequently transplanted organ worldwide. In France, 17 277 patients were on the waiting list in 2021 and only 3252 transplantations were performed. This organ shortage has an adverse impact on the survival of patients with chronic kidney disease. To improve access to transplantation, an increasing proportion of grafts have come from donors with expanded criteria. Expanded criteria donors (ECDs) are defined as donors who are brain dead and aged >60 years or from 50 to 59 years with two of the following criteria: death from cerebrovascular cause, renal insufficiency with creatinine above 150 µmol/L or a medical history of hypertension. This represented 56% of kidney transplants from brain-dead donors in France in 2021. Their use is associated with a higher rate of primary non-function and delayed graft function than that of standard criteria brain-dead donors.1 One of the major reasons for these differences in prognosis is ischaemia/reperfusion injury (IRI). The optimisation of donor management, reduction of cold ischaemia time and use of hypothermic perfusion machines have been progressively implemented in the organ procurement process to improve prognoses.2
Organ enhancement by machine perfusion is a strategy of particular interest3 and is widely used in clinical practice.1 Moreover, additional promising strategies are currently under development. For example, the use of oxygen carriers in the preservation solutions of machine perfusion for brain-dead donor grafts could decrease the rate of delayed graft function, independently of the cold ischaemia time,4 and perfusion with extracellular vesicles and stem cells may decrease the impact of ischaemia–reperfusion and promote tissue repair.5–9
Mineralocorticoid receptor antagonists (MRAs) appear to be a promising and simple way to prevent IRI-induced acute renal failure. In preclinical studies, Barrera-Chimal et al investigated the prevention of acute renal failure in rodents and pigs related to IRI by MRAs, which block the Rac1 portion of Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase located in vascular smooth muscle cells, thus inhibiting the synthesis of reactive oxygen species (ROS). ROS modify the endothelin B receptor and prevent endothelial nitric oxide synthase activation and nitric oxide release. MRAs would therefore increase nitric oxide bioavailability and thus vasodilation.10 11 In addition, MRAs given prior to ischaemia prevent chronic renal failure after an episode of ischaemia in rodents and pigs. The increase in interleukin (IL)-4 secretion stimulated by MRAs induces a change in the macrophage profile towards an M2, anti-inflammatory profile. The secretion of transforming growth factor-β, tumour necrosis factor-α and other proinflammatory mediators is decreased, as well as the aberrant repair that leads to chronic renal failure.12 In humans, the use of MRAs has been shown to be associated with a decrease in oxidative stress after renal transplantation, without any evidence, to date, of a diminution in the prevalence of acute or chronic renal failure.13 However, this study was conducted on patients receiving living donor transplants, with a very low prevalence of spontaneous graft dysfunction. A randomised controlled, double-blind trial, EPURE-TRANSPLANT (NCT02490904), is ongoing and aims to evaluate the impact of the administration of eplerenone in the perioperative period among kidney graft recipients receiving a graft from an ECD. The primary outcome is graft function at 3 months. The results are not yet available.14
MRAs are frequently used as antihypertensive or diuretic drugs and are recommended for chronic cardiac failure with reduced ejection fraction.15 Data on the use of MRAs in intensive care patients outside the indications described in the marketing authorisation are scarce. Clinical studies in perioperative or brain-injured patients have reported inconsistent haemodynamic tolerance of MRAs.16–19 Overall, the use of MRAs causes a small increase in potassium levels, which in some cases has led to the discontinuation of treatment.20
During the phase of brain herniation in brain-dead donors, haemodynamic instability may occur. These episodes are related to catecholaminergic discharge21 22 followed by profound depletion. A cytokine ‘storm’ is associated, resulting in systemic inflammatory response syndrome. Microcirculatory dysfunction may occur with IRI.23
Given the potential haemodynamic effects of MRAs, we sought to evaluate the tolerance of such treatment in brain-dead donors already at risk of haemodynamic instability before evaluating the effectiveness of their administration in this population on the viability of renal grafts. Our hypothesis is that potassium canrenoate is non-inferior to placebo in maintaining haemodynamics in brain-dead donor patients and may improve graft function in kidney transplantation.
Methods and analysis
Trial design
The trial protocol followed the recommendations of the Standard Protocol Items: Recommendations for Interventional Trials (https://www.spirit-statement.org).
This single-centre study is a prospective, randomised, controlled, double-blind, two-arm, parallel group, phase II clinical trial carried out at the University Hospital of Nancy, France. Brain-dead patients for whom kidney procurement is considered are eligible for inclusion if they fulfil all inclusion criteria without the presence of exclusion criteria. The included patients are randomised (1:1) and stratified by age to receive either 200 mg potassium canrenoate or placebo every 6 hours until aortic cross-clamping in the operating room.
Participants
Inclusion criteria
Patients hospitalised in one of the intensive care units (ICUs) of the University Hospital of Nancy, France are eligible for inclusion if:
They are aged >18 years.
A diagnosis of brain death is made by two flat and unreactive electroencephalographs made at a 4-hour interval and for 30 min each or by cerebral angiography showing the absence of intracranial circulation according to the 2005 decree and the French public health code.24
It is planned to remove at least one kidney.
Vasopressors did not vary more than 1 mg/hour the hour before inclusion and the dose is lower than 7 mg/hour.
They are considered to be euvolemic.
They are affiliated with a medical care system.
Written consent by testimony from a family member or a person of trust has been obtained in accordance with the French public health code (L. 1121-14).
Exclusion criteria
Patients are excluded if they fulfil any of the following criteria:
Received potassium canrenoate in the last 48 hours.
Presence of MRA in their usual medications.
Potassium >5.5 mmol/L.
Any contraindication to multiorgan retrieval (infection, cancer).
Refusal of organ removal expressed by the patient (national register of refusals or reported by the family).
Probable inability to remove/transplant the kidneys: urological or nephrological history, pre-existing chronic renal failure, morphological abnormalities of the kidneys or renal trauma.
Inclusion in another interventional drug clinical trial.
Known hypersensitivity to potassium canrenoate and/or trometamol.
Severe renal insufficiency (creatinine clearance <30 mL/min/m2).
Severe atrioventricular conduction disorders.
End-stage hepatocellular insufficiency.
Pregnant, parturient or breastfeeding women.
Individuals deprived of their liberty by a judicial or administrative decision.
Minors (not emancipated).
Adults subject to a legal protection measure (guardianship, curatorship, safeguard of justice).
Individuals under psychiatric care without consent.
Participant recruitment
Brain-dead donor patients admitted to one of the ICUs of the Department of Anesthesia and Intensive Care of the University Hospital of Nancy, France in the context of multiorgan procurement are considered for inclusion.
Inclusion and randomisation are performed when the patient arrives to the ICU in charge of brain-dead donors in the absence of contraindication and after obtaining written consent by testimony from a member of the family or a trusted person in accordance with the French public health code.
Randomisation
Randomisation and treatment allocation are processed via the secure web platform CleanWeb and stratified according to an age ≤52 or an age >52 years, which is the median of brain-dead donors in our centre.
Remote access to the randomisation platform is provided to authorised users only. Individual and renewable passwords are provided to users with specific access rights depending on their role in the study.
Care providers and participants are unaware of the allocation.
Intervention
Timing of administration of the study treatment
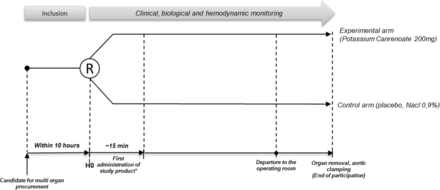
The drug or placebo is prepared by another department in packaging that makes it impossible to distinguish the potassium canrenoate from the placebo. It is administered to the patient within 10 hours after the diagnosis of brain death and before the patient is transferred to the operating room. If the patient is still in the ICU 6 hours later and the serum potassium concentration is <5.5 mmol/L, a second dose of the treatment is administrated (figure 1).
Study design. *Second administration of study product 6 hours after H0, if the patient is not admitted to the operating room. R, randomisation.
Premature discontinuation of study treatment
The treatment is not administrated if either the serum potassium concentration is >5.5 mmol/L or if there is haemodynamic instability, defined by an increase in the dose of vasopressor to >5 mg/hour. Nevertheless, the patients are monitored until the end of study participation.
Appropriate administration of treatment
The unused and used treatments are returned to the local pharmacy to verify compliance of the administration according to the protocol.
Unblinding modalities
The investigators and study sponsor are the only individuals authorised to unblind the randomisation. The person in charge of vigilance can unblind independently of the investigator, without informing him of the result. The unblinding can be performed at any time, if necessary, by the service preparing the treatment at the University Hospital of Nancy, which knows the arm of randomisation.
Concomitant medications
During participation in the study, the patients are not allowed to receive other MRAs. Due to its nephrotoxicity, hydroxyethyl starch is forbidden. All treatments used are reported.
Study outcome
Primary outcome
The primary outcome is a hierarchical event composite outcome, as described by Felker and Maisel in 201025:
Circulatory arrest before organ procurement.
Inability to perform renal procurement.
Average hourly dose of epinephrine/norepinephrine between randomisation and departure to the operating room.
Average volume of colloids/crystalloids between randomisation and departure to the operating room.
These data are collected from the patient’s medical record and anonymised through identification numbers.
Secondary outcomes
The secondary outcomes are meant to evaluate the impact of the treatment in protecting the graft against IRI and will include:
Vital status of the kidney graft recipients and the serum creatinine concentration (in μmol/L), with the estimated glomerular filtration rate (GFR), according to CKD-EPI (in mL/min/1.73 m2), at 3 months after kidney transplantation.
Percentage of kidney graft recipients dependent on dialysis and/or with an estimated GFR <20 mL/min/1.73 m2 at 3 months.
Vital status of kidney graft recipients at 3 months.
Vital status of kidney graft recipients and serum creatinine concentration (in μmol/L), with the estimated GFR according to CKD-EPI (in mL/min/1.73 m2), at 1 year, 3 years and 10 years after the transplant.
The results of the secondary outcomes will be collected via the French database of organ recipients with full anonymisation (CRISTAL Database, Agence de la Biomédecine).
Follow-up of participants
All patients are hospitalised in an ICU that oversees brain-dead donors. Their vital parameters (invasive arterial blood pressure, heart rate, photoplethysmography, etc) are continuously monitored, as well as their cardiac output, determined by arterial pulse contour analysis (Mostcare, Vygon, Ecouen, France). Blood gas analysis is performed every hour for potassium monitoring. Kidney function is evaluated by diuresis and creatinine clearance measured over a 4-hour period. The prescription and dose of medications, vasopressor amines and crystalloids/colloids are followed during hospitalisation in the ICU. The monitoring is continued until the aortic clamp (figure 2).
Study flow chart: randomisation, timing of administration of the study treatment and primary outcome measurement. R, actions carried out specifically for research purposes; S, actions carried out in the context of care.
Adverse effects
Known adverse effects of MRAs are an increase serum potassium levels and alterations of creatinine levels urine output. Any other potential adverse events are also recorded. The recording of serious adverse events respects Good Clinical Practice and French law. Thus, the physician informs the sponsor as soon as possible. In addition, the investigator oversees the recording of all adverse effects in the case report form.
Statistical analysis
Number of patients and statistical power
We hypothesise that no cardiocirculatory arrest will occur before organ harvesting and that all organ procurement will be performed. We expect a mean hourly norepinephrine/epinephrine dose of 1.18 mg/hour in the control group vs 1.42 mg/hour in the canrenoate group (+20%), with a common SD of 0.92 mg/hour and a mean crystalloid volume of 1 L in the control group vs 1.2 L in the canrenoate group (+20%), with a common SD of 2 L.
Thirty-six randomised patients are required to obtain at least 80% power with a two-sided 5% alpha risk. If there is 5% cardiocirculatory arrest, the power will still be >80%.
Primary and secondary outcomes
The primary outcome is a prioritised event composite outcome. It will be analysed using a parametric test (Student’s t-test) based on ranks.
For the secondary outcomes (numbers 1, 2, 3), the mean values and CI of the groups will be compared. A logistic regression model will also be performed after adjustment on variables identified as being significantly associated with the randomisation arm in the univariable analysis. For secondary outcome number 4, a similar strategy will be used, except with survival modelling.
Ethics and dissemination
Ethics committee approval
The study will follow the Helsinki principles, French regulations and Good Clinical Practice. The French health authorities (Agence Nationale de la Sécurité du Médicament) approved this trial on 10 August 2020. An ethics committee approved this trial on 21 December 2020 (Comité de Protection des Personnes: CPP Ouest II-ANGERS). The trial was registered in the European Clinical Trial Database (EUDRA-CT: 2020-003285-40) and with ClinicalTrials.gov (NCT04714710).
Information and consent
Given the state of brain death of the donor patient and after being informed about the study, the investigator seeks the testimony of the family or a trusted person about what the patient would have accepted in view of his or her convictions or any discussions about the research, in accordance with the French public health code. Without the testimony of the family or a trusted person in favour of the research, the patient cannot participate in the study and is followed up in accordance with the usual care.
Patient and public involvement
None.
Data quality and regulatory issues
Monitoring during the study
Study monitoring is performed by the sponsor (Direction de la Recherche et de l’Innovation, CHRU de Nancy, France) to ensure compliance with the protocol and applicable regulations, that good clinical practices are maintained, and that data are collected in a timely, accurate and complete manner. The monitoring is conducted in accordance with the specific monitoring plan of the research.
Specific boards of the research project
Steering committee
The steering committee consists of the clinical initiators of the project: Professor Philippe Guerci (anaesthesiologist and intensive care physician), Dr Sophie Girerd (nephrologist), the biostatistician/methodologist in charge of the project (Professor Nicolas Girerd), Professor Luc Frimat (nephrologist), Dr Hélène Gregoire (Organ and Tissue Procurement Unit of the CHRU of Nancy) and representatives of the sponsor.
It establishes the general organisation and progress of the research and coordinates information. It also determines the methodology, decides what to do in unforeseen cases and monitors the progress of the research, particularly in terms of tolerance and adverse events.
Independent monitoring board
An independent monitoring board (IMB) has been set up. The IMB has an advisory and decision-making function when called upon by the sponsor on medical issues, such as tolerance and adverse events. It is made up of three people independent of the research and includes a kidney transplantation expert, an anaesthesiologist-intensive care physician and a methodologist-statistician. The selection of the IMB members was made by the coordinator and the sponsor, based on their expertise in the medical field and clinical trials. Members of the IMB sign a commitment to participate and not to have any relationship with the study, the study product, the sponsor or the investigators. The IMB will meet before the beginning of the study and then annually and exceptionally in the event of a new development or a request from the coordinating investigator or the sponsor.
Data management and interpretation
Data management, statistical analysis and data interpretation are being carried out by the clinical investigation centre (CIC-P 1433 of Nancy). Study data are remotely captured via electronic case report forms (eCRFs) designed using Ennov Clinical Electronic Data Capture (EDC) software V.7.5. Remote access to the eCRFs, via a secured web browser (https), is provided to authorised users only. Individual and renewable passwords are provided to users with specific access rights depending on their role in the study. Data inconsistencies are resolved according to the data validation plan implemented in the EDC. Following data review, the database will be locked and provided as SAS V.9.4 tables to the statistical team for analysis.
Dissemination
The results of the trial and other evaluation findings will be presented at scientific meetings and submitted for publication in international peer-reviewed journals. Upon completion of the trial and after publication of the primary manuscripts, data requests can be submitted to the primary investigator.
Discussion
Context of organ shortage and mandatory use of ECD grafts
The number of patients with end-stage renal disease has dramatically increased over the last decades.1 Kidney transplantation is the best therapeutic option for life expectancy, as well as for quality of life and limiting costs.26–32 Given the growing number of patients on the waiting list and the organ shortage, the need to improve the results of kidney transplantation from ECDs is urgent. Indeed, 56% of kidney grafts from brain-dead donors originate from ECDs. However, the rate of primary non-function or delayed function is greater than that with living donors or donors with standard criteria.1
Impact of MRA for prevention of IRIs
IRI combines disturbed regulation of vascular tonus, inflammation and structural damage by apoptosis or necrosis. In addition, repair is often maladaptive, with capillary rarefaction and interstitial fibrosis. The repetition of these aggressions or their occurrence in a kidney with reduced nephrotic capacity is a step towards chronic renal failure, or in the case of a graft, delayed function, primary non-function or reduced survival.33
Beyond its classic impact on renal sodium/potassium homeostasis and blood pressure control, mineralocorticoid receptors are also present on endothelial cells and vascular smooth muscle cells. Activation of the mineralocorticoid receptors in the vascular tree promotes vascular remodelling toward fibrosis and calcification.34
The mineralocorticoid receptor is also expressed on myeloid cells and its antagonisation (pharmacological blockade or genetic inactivation) promotes expression of the IL-4 receptor. Overexpression of these receptors guides the polarisation of macrophages towards an M2 phenotype, consisting of an anti-inflammatory profile and a pro-angiogenic subtype.12
In pigs and rodents, Barrera-Chimal et al demonstrated that MRAs prevent the onset of acute renal failure following ischaemia, as well as progression to chronic renal failure.10–12 35 In clinical studies, the impact of MRA administration in the setting of kidney transplantation has not yet been proven. However, a study conducted in 2018 consisted of the administration of spironolactone to living donor kidney graft recipients in the preoperative and postoperative periods. The authors reported a decrease in urinary excretion of HSP72 and 8-hydroxylated-guanosine, markers of oxidative stress, in the spironolactone-treated patients.13 In another study in the same population, the authors showed that the administration of spironolactone decreased the urinary excretion of H2O2.36 The EPURE-TRANSPLANT (NCT02490904) trial will provide answers about kidney transplants from ECDs. Nevertheless, the administration of MRAs to the donors could further improve the prognosis of kidney grafts, given the haemodynamic disturbances/instability that occurs during the period before organ procurement.
The state of brain death, a relevant moment to act
Brain-dead donors are hospitalised in intensive care for several hours. This time, used for the analysis of the collectability of the organs and their distribution, is crucial for organ optimisation. For example, the administration of methylprednisolone to the donor increases the long-term success of lung transplants and is an integral component of the American and British recommendations.37 38
Concerning MRAs, preclinical studies have shown the relevance of their administration in the prevention of ischaemic events.35 39 40 Thus, the period between brain death and organ procurement is the most suitable for the administration of therapeutics aiming to reduce IRI.
Justification of the choice of MRA and dosing
According to the cause of brain death, there may be a delay in gastric emptying or a functional ileus, making the absorption of drugs uncertain. To avoid such uncertain absorption, we chose to use an MRA that can be administered via the intravenous route. The only MRA available for administration by this route is potassium canrenoate. This drug, which is frequently used in our department, is administered at a dose of 200 mg per dose, as described in the summary of the product characteristics.
In addition, once administered, potassium canrenoate is metabolised to canrenoic acid, with a half-life of 0.07 hours, to canrenone, an active molecule with a half-life of 0.8 hours, and to glycuronide of aldadiene, an inactive compound with a half-life of 43 hours.41
Given these pharmacokinetics, it was decided to readminister the product or placebo after 6 hours if the patient was still present in our service.
Justification of primary outcome
In the state of brain death, except for contraindications, the main factor that can prevent organ procurement is major haemodynamic instability and, by extension, cardiocirculatory arrest. Moreover, haemodynamic instability is the cause of additional aggressions for the grafts. MRAs are diuretics and can be used as antihypertensives42 Thus, it seems necessary to evaluate their safety in these brain-dead donors prior to evaluation of the effectiveness of MRAs on the reduction of IRI. Very few studies are available on the impact of their administration in ICU patients. Suyagh et al investigated the pharmacokinetics of potassium canrenoate in resuscitated children but did not address the question of tolerance.43 The administration of MRAs in addition to mannitol to 56 brain-injured patients by Bilotta et al showed better potassium retention and a decrease in cardiac arrhythmias.19 In the perioperative setting, potassium canrenoate did not lead to any modification of cardiac output, arterial pressure or the dose of vasopressors.15–18 44
To make an integrative assessment of tolerance, a hierarchical composite endpoint will be used. This strategy has been described previously45 and resembles the win-ratio approach that recently gained attention in the field of cardiovascular medicine (ie, in the EMPULSE trial).46 The use of this integrative outcome will enable an integrative yet granular evaluation of the practical impact of MRA treatment on the organ swabs and haemodynamic tolerance.
Clinical implication
We hypothesise that the use of MRAs in brain-dead patients is haemodynamically well tolerated. If this is confirmed, we could focus on the effectiveness of MRAs in the reduction of IRIs of the kidney grafts. To this end, a multicentre study based on the principal hypothesis of an improvement in the survival and function of the grafts removed from brain-dead patients, more precisely, those with expanded criteria, will be conducted. As mentioned before, the administration of a drug to prevent IRI to the donor could be of particular interest, given that a large part of any injury to the organ may occur before its procurement. Most of the interventions under development focus on the prevention of IRI after organ procurement. Nevertheless, a combined approach could be the most effective/promising.
Trial status
The first patient was included in August 2021 and enrolment is ongoing. To date, 29 patients have been included.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors FJ, PG and SG conceptualised the idea for the study. PG, NG, XL, KD, LB and LM contributed to the study conception and design. PG is the principal investigator of the study. LB contributed to drafting the manuscript. All authors have revised the manuscript. All authors have read and approved the final submitted version of the manuscript.
Funding This study was supported by a grant from the French Eastern Interregional Group of Clinical Research and Innovation (GIRCI Est; appel à projet «jeunes chercheurs», APJ 2019).
Competing interests LB, SG, XL, KD, FJ and LM report having no competing interests. PG reports receiving consulting fees from Edwards LifeScience and AOP Health. NG received honoraria from AstraZeneca, Bayer, Boehringer, Lilly, Roche Diagnostics, Novartis and Vifor.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.