Article Text
Abstract
Introduction Urinary incontinence (UI) is associated with increasing age and is more frequently experienced by women. Despite 40% prevalence in the community, little is known about the prevalence/incidence of UI in older women during hospital admission. UI during hospital admissions, within this group, has also been under-researched in terms of its relationship to specific clinical conditions and mortality rates. Given that UI has serious implications for both patient care and women’s general health and well-being on discharge, this protocol describes a planned research project which aims to determine mortality, morbidity, prevalence and incidence of UI in older women (≥55 years) during hospital admission to inform nursing practice. Additionally, it aims to explore the experience of nurses who deliver women’s care.
Methods and analysis This is an explanatory mixed-methods study consisting of two phases: (1) retrospecitive analysis of electronic patient care records (EPCR) to determine prevalence/incidence of UI, clinical conditions most likely associated with UI and any associations between UI and death, (2) nurse interviews to explore views, knowledge and perceptions of performing the nursing assessment and providing care for older women (≥55 years) with UI during admission. EPCR will be gained from a National Health Service (NHS) teaching hospital. Nurse interviews will be conducted with nurses from an alternative but similar-sized NHS hospital.
Ethics and dissemination Ethical approval is provided by the University of Salford Ethics Committee and regulatory approval by the NHS Health Research Authority (Integrated Research Application System project ID: 303118). Local NHS trust approval to access electronic care records for the purposes of analysis of anonymised data has been provided by one of the two collaborating NHS hospitals. Findings will be disseminated through open-access geriatric or urogynaecology journals and presented to relevant stakeholders at local, national and international meetings including scientific meetings such as the UK Continence Society and International Continence Society.
- Urinary incontinences
- UROLOGY
- Urogynaecology
- Bladder disorders
- Quality in health care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The explanatory mixed-methods design allows greater depth of interpretation and understanding of the quantitative data through integration of the qualitative interview themes.
Analysis of electronic inpatient records will provide insights of the prevalence/incidence of urinary incontinence for older women admitted to hospital and outcome during routine care and enable broader generalisability.
Use of routine electronic nursing assessments across all inpatient wards at a large National Health Service teaching hospital allows insight of nursing practices across a large amount of patient interactions with older women, thereby reducing the impact of local variability of practice and cultural influences.
Electronic care records data are from an organisation different to the organisation where interviews will be conducted and therefore it is possible that nurses’ experiences of conducting nursing assessments and knowledge may differ, or not reflect practices at the hospital providing electronic data.
Frailty data are calculated using the Leicester Frailty Score, which requires an admission to hospital within the last 24 months within the algorithm; this may result in potential underestimation of the proportion of individuals with frailty.
Background
Urinary incontinence (UI) impacts approximately 33% of older (≥55 years) women1 and can lead to a reduction in their quality of life, reduced psychological health, lack of confidence, feeling of reduced sexuality and societal exclusion.2 Some women report feeling embarrassment and a sense of taboo related to their continence issues and may not seek help or discuss the matter with others including their partners.2–6 Older women are also less likely to be referred to continence care by their general practitioner than younger women.7
The majority of empirical UI research has been conducted in community rather than hospital inpatient settings, and little is known about the prevalence and incidence of UI in older women during hospital addmissions. Additionally, research conducted in community populations has demonstrated that UI is not correlated with increased mortality or significant morbidity. Our review indicated that there is no published literature investigating older women’s UI and mortality rates in secondary care populations.8 It is also not clear what disease areas or clinical problems increase the incidence or whether UI impacts morbidities, such as frailty, for these women. However, it is known that individuals who are at the end of their life, or in receipt of palliative care, are more likely to experience UI, although they are more likely to be in receipt of catheterisation for their UI.9
Causes of UI for older women include a weak/damaged pelvic floor, pressure on the bladder due to obesity10 and/or physiological changes due to menopause. Other issues implicated are an overactive bladder or constipation, drinking alcohol or caffeine, concentrated urine and frequent urinary tract infections.11 Certain medications, such as diuretics, anti-depressants, hormone replacement therapy, ACE inhibitors and sedatives, may also cause problems.12 Women of advancing age are also at greater risk of UI due to associated conditions being more likely to occur in older age; despite this, UI is not a normal part of ageing.13 14
Some clinical conditions (eg, arthritis, stroke, Parkinson’s disease, multiple sclerosis, dementia) that limit a woman’s mobility and ability to easily reach toileting facilities may increase the likelihood of UI. Women with comorbid conditions such as neurological conditions (eg, Parkinson’s disease, multiple sclerosis), diabetes, heart failure, hypertension and respiratory disease may also be more likely to experience UI particularly during episodes of exacerbation of their symptoms.15 16 Studies have shown that UI in older women is a contributing factor to both falls and the development of pressure ulcers.17–19 Moisture and/or UI, along with other factors such as immobility, friction, reduced nutritional state, age and comorbid conditions, are all known contributory factors to development of pressure ulcers.19 20 Likewise, immobility and difficulty accessing toileting facilities in a timely way have also been linked to falls.21 22 In turn, both falls and pressure ulcers are associated with frailty and increased mortality in older adults.23
A wealth of patient care information is held by National Health Service (NHS) hospitals, recorded through electronic patient care records (EPCR).24 These records include nursing assessments, which help nurses to plan and implement person-centred care. The assessment is based on the activities of daily living25 and includes a person’s continence status. Assessments are routinely undertaken on admission and repeated either weekly or when the patients’ health condition, clinical status or circumstances change (ie, post-surgery or unplanned clinical event). The assessment includes: (1) their normal continence status prior to admission, (2) continence at the point of admission and (3) repeat continence assessment as previously described. Using EPCR is a useful means to better understand mortality and morbidities (such as frailty and pressure ulcers) and whether there is an association with UI for older women. Prevalence and incidence of UI in older women may also be better understood.
There is a need to explore the size of the problem in terms of the number (prevalence and incidence) of women (≥55 years) who experience UI during hospital admission and whether their mortality is impacted while an inpatient. Greater understanding is needed of nurses’ (inclusive of the wider nursing team) views, knowledge and perceptions of UI when conducting nursing assessments and caring for women who experience UI during an inpatient admission. This information will inform development of a nursing educational resource which will be tested empirically in future research. Such a resource may help support nurses and others when caring for patients and informing women of helpful self-management techniques and signposting them to other services. The following protocol describes a study to investigate UI in older (≥55 years) women during secondary care admissions and explores nurses/clinical support workers’ experiences of delivering related care.
Methods and analysis
This is a mixed-methods study using an explanatory26 design (figure 1) that includes two phases: phase 1—quantitative, retrospective study using electronic nursing and medical patient records to determine prevalence, incidence, mortality and morbidities associated with UI in older women (≥55 years) during a hospital admission; phase 2—qualitative interview study to explore the experience of nurses delivering women’s care.
Mixed-methods explanatory design (quantitative emphasised).26
The data will be collected between 1 November 2019 and 28 February 2023.
The quantitative and qualitative phases will be undertaken consecutively, with qualitative findings being used to add explanation and interpretation to the quantitative findings. Integration of findings from the two phases will lead to greater understanding of women’s UI during a hospital admission.
Phase 1
This phase of the study will be a retrospective study of women aged 55 years or over who were admitted to a large NHS hospital between 1 November 2019 and 29 February 2020. This phase will use electronic inpatient data, including medical diagnoses and nursing assessment to determine:
The prevalence of UI on admission and incidence of women (≥55 years) becoming incontinent (new cases over time) during an admission to hospital.
Which clinical conditions and/or health-related risk factors (based on medical diagnoses using International Classification of Diseases 10th revision (ICD-10) codes) are associated with UI, after accounting for confounding factors, for older women admitted to hospital.
Mortality rates and where UI is associated with mortality after controlling for confounding factors, for older women admitted to hospital.
Methodology
De-identified electronic data associated with patient records will be extracted from the data warehouse at a large NHS hospital in the north of England. Women who have withdrawn consent for the use of their electronic health records for research and are registered with the NHS Digital national data opt-out service will be excluded from the analysis. To do so, a list of NHS numbers for all possible ‘participants’ will be provided to NHS Digital via the secure messaging system (MESH). NHS Digital will return a list of NHS numbers for patients who are opted in; data associated with those who have opted out will not be extracted from the data warehouse. This process will be conducted by analysts from the hospital’s Research and Innovation department.
The de-identified data will then be securely transferred to researchers at the University of Salford using secure (encrypted) NHS mail in line with NHS Digital guidance (https://digital.nhs.uk/services/nhsmail/guidance-for-sending-secure-email). Data will be stored on an access-controlled file server. Data will be analysed by researchers at Salford University.
Data will be included for all women ≥55 years. This age group has been selected as they have been demonstrated to be more likely to experience UI than women under 55 years.14 27 28
In order to estimate the sample size needed to achieve sufficient accuracy in estimating prevalence, we used the following formula:
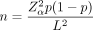
where Zα is the two-tailed Z-value from the confidence level, p is the expected proportion of patients that will be recorded as having UI and L is the precision on the expected proportion (see SRUC: Epidemiology Resources sample size estimator app at https://epidemiology.sruc.ac.uk/shiny/apps/samplesize/). Based on literature describing community-based prevalence, we estimated a prevalence of 50%. Based on this, the sample size needed will be 2401 in order to have 95% confidence that the ‘true’ prevalence of incontinence is within 2 percentage points of this (ie, prevalence between 48% and 52%).
Women will initially be separated into four cohorts (identified from the electronic nursing assessment for continence and ICD-10 codes):
Continent: this will include all individuals who were recorded as ‘continent’ on all nursing assessments during the admission. Individuals with any ‘incontinent’ ICD-10 codes or individuals recorded as having a catheter on any nursing assessment will be excluded from this group.
Incontinent: this will include individuals recorded as incontinent of urine in any nursing assessment during admission and individuals with a UI ICD-10 code in medical diagnosis variable during admission. Individuals who are also recorded as having faecal incontinence on any nursing assessment, or individuals recorded as having a catheter on any nursing assessment, will be excluded from this group.
Double incontinent: this will include individuals recorded as incontinent of both urine and faeces on nursing assessments. Individuals recorded as having a catheter on any nursing assessment will be excluded from this group.
Indwelling catheter: this will include individuals recorded as having a urinary catheter on any nursing assessment.
These cohorts will be used to establish prevalence and incidence. Data will not be included for men, women under 55 years, women admitted for surgical procedure directly related to UI or women who have registered through the national data opt-out.29
After prevalence and incidence have been established, additional analysis to establish associations between UI, health conditions and mortality will use only the incontinent and continent cohorts. For this analysis, women admitted with double urinary and faecal incontinence, and women treated at any point during their admission with indwelling catheter for UI will not be included.
Data will be extracted adhering to the priori inclusion/exclusion criteria, for the time period (1 November 2019–29 February 2020). This time period was selected because the electronic nursing assessment was embedded across the hospital on all inpatient wards during this time period, increasing the likelihood of a full dataset. Data beyond February 2020 will not be included due to changes in the nursing assessment that occurred during the COVID-19 pandemic. Data related to both the initial admission nursing assessment and any repeated nursing assessments will be retrieved. The following variables will be extracted:
Demographics: demographic data collected from electronic patient records will include age at admission and ethnicity. Deprivation deciles will also be calculated using the Index of Multiple Deprivation (IMD). This will involve mapping a patient’s postcode of residence at the time of admission (or any address recorded closest to the admission date) to Lower Super Output Area (LSOA). LSOAs will then be assigned to an IMD decile.
Clinical and health data: clinical and health-related data will be extracted from electronic records for use within analysis. These will include:
Body mass index (BMI): this will be calculated through patient height and weight data and used to categorise patients into four groups: underweight, healthy weight, overweight and obese.
Mobility: this will be gained from nursing assessments and used to catagorise patients into five categories: independent, poor mobility, history or risk of falls/presents with acute fall, immobile and dependent for all movement and repositioning.
Frailty risk: the Hospital Frailty Risk Score30 will be calculated by using ICD-10 diagnosis codes for all inpatient admissions that occurred 2 years before the date of admission. Scores will be assigned to a selection of three-character ICD-10 codes, depending on their association with frailty and the scores summed for each individual. The scores will then be used to categorise patients into four categories: low, intermediate, high and unknown. The ‘unknown’ category will include women who do not have inpatient admissions recorded within the 2 years before the current admission.
Inpatient diagnosis: primary and secondary diagnosis data will be collected using ICD-10 codes.
Pressure ulcer risk: presence (or absence) of a pressure ulcer risk will be collected based on nursing assessment data.
Length of stay: the length of stay for any admission will be collected.
Additional data including information on surgical interventions relating to urinary incontinence will also be collected for exclusion purposes. This will be gained through Classification of Interventions and Procedures-4 codes, a fundamental information standard used by NHS trusts to code procedures.31
Mortality: fact of death records will be extracted to assess mortality rates associated with incontinence. This will include deaths during admission, deaths within 30 days of discharge and deaths within 3 months of discharge.
Statistical analysis
Prevalence and incidence: descriptive analysis will be undertaken to determine:
Prevalence of incontinence on admission through the count and percentage of women falling within the four continence cohorts: (1) continent, (2) incontinent, (3) double incontinent and (4) those with an indwelling catheter.
Incidence of women (≥55 years) becoming incontinent of urine during admission will be determined through counts and percentages over time.
Incontinent versus continent group characteristics: the study population will be described using descriptive statistics by presenting counts and percentages, and/or means and SDs (as appropriate) for both the demographic data (age, ethnicity, deprivation status) and health-related data (BMI, mobility, frailty risk, pressure ulcer risk). Group comparisons using t-tests and/or Χ2 tests will be performed to assess differences between the incontinent and continent groups.
Health characteristics associated with UI: to determine which health characteristics are associated with UI, binary logistic regression will be carried out with UI (yes/no) as the outcome. Characteristics included in the model as independent variables of interest will be frailty, mobility and BMI. Based on our clinical knowledge and the literature, we have determined age to be a potential confounding factor of this relationship and so this will also be included as a covariate in the model. From these models, we will report the adjusted ORs with accompanying 95% CIs and p values (p<0.05 will be considered significant for all analyses).
Clinical conditions: primary and secondary diagnosis data based on ICD-10 codes will be described using descriptive statistics by providing counts and percentages. Binary logistic regression will be carried out with UI (yes/no) as the outcome and clinical conditions will be selected based on current literature (Parkinson’s disease, multiple sclerosis, stroke, dementia, diabetes, heart failure, etc). Confounding factors such as age and BMI will also be included in the model as covariates. From these models, we will report the adjusted ORs with accompanying 95% CIs and p values (p<0.05 will be considered significant for all analyses).
Mortality: to determine whether UI is associated with mortality, data will be analysed by all deaths, deaths within 30 days of discharge and deaths within 3 months of discharge. For each time period, the following will be reported:
Total deaths: this will be described using counts and percentages and the number of deaths for the incontinent and continent cohorts.
Mortality rates: this will be calculated by dividing the total deaths by total person-years at risk for the whole cohort from admission to the end of the time period. Person-years will be calculated from summing time from admission to either death (if the death occurred before the end of the follow-up period) or to the end of the follow-up period for each individual.
Kaplan-Meier curves: this will be used to show the overall survival probability from the day of admission.
HRs: these will be created using Cox regression modelling for deaths with the continent group (incontinent vs continent) as the independent variable of interest. Based on our clinical knowledge and the literature, we have determined the following to be potential confounding factors of this relationship: age, BMI, mobility, falls, pressure ulcers, urinary tract infection, Parkinson’s disease, multiple sclerosis, stroke, dementia, diabetes, heart failure, hypertension, myocardial infarction, chronic obstructive pulmonary disease and frailty. How many of these confounding variables we will be able to include will be determined by the number of ‘events’ (deaths) in the model. If all cannot be included, they will be ranked by likely magnitude of their confounding influence to determine their inclusion in the model. Accompanying 95% CIs and p values (p<0.05 will be considered significant for all analyses) will also be reported.
Missing data: data fields will be checked for missing or incomplete data and where more than 40% of patient data are missing, they will not be included in the analysis. To ensure meaningful analysis, minimum data requirements are set so that at a minimum, the following data will be available for each participant: age, gender, continence status, catheterisation status, mortality status, clinical diagnosis and pressure ulcer risk.
Phase 2
Phase 2 of the study will use qualitative interviews to gain an understanding of nurses’ views, knowledge and perceptions of providing care for older women with UI during hospital admission, and explore their use of nursing assessments. For pragmatic reasons, nurses to be included in the interviews will be employed at a hospital different to the hospital providing analysis of the women’s electronic patient nursing records.
Methods
Semistructured interviews will be conducted on one occasion with 15–20 (to allow for withdrawal) nurses working on a variety of female or mixed inpatient wards at a large, northern NHS tertiary hospital. Interviews will be with those from the wider nursing team both registered (UK Nursing and Midwifery Council) and non-registered nurses.
Ward managers will be contacted, by the researchers, and asked for permission to attend ward meetings to discuss the study with staff; both written and verbal information will be provided to potential participants. Informed consent will be taken prior to interviews taking place.
The interview schedule will be informed by the literature and findings from the quantitative phase of this study. A draft schedule will be developed in consultation with patients with lived experience. It is likely to include participants’ (nurses) experiences of delivering care to older women experiencing UI, their views towards women's UI, as well as their knowledge, education and training pertaining to women’s UI. It will then be piloted and potential adaptations made.
Concurrent data collection, data analysis and purposeful participant selection will take place with the themes from interviews influencing selection of the next participant. Interviews will take place remotely or at the participant’s workplace.
Qualitative analysis
The framework approach,32 along with thematic analysis (including induction and deduction), will be used to understand the data. Framework is underpinned with five interconnected stages including familiarisation, identifying a thematic framework, indexing, charting, and mapping and interpreting. Analysis will involve a series of interconnected stages enabling the researcher to move back and forth across the data until a coherent account emerges.32 This results in the constant refinement of themes which may lead to the development of a conceptual framework.
Mixed-methods integration of data
The findings from each of the two phases will be synthesised to draw overall conclusions. The integration of mixed-methods data may confirm and elaborate the findings from phase 1 and phase 2.
The data from this study will inform adaptation of an existing self-help guide developed by members of our research team, for community-dwelling women (≥55 years) experiencing UI.33 The intention being that the Fu et al33 self-help guide will form the basis of an educational intervention for nurses who provide care to women experiencing UI during an inpatient admission.
Patient and public involvement
This study has been informed throughout by women with lived experience of UI and clinicians who work in the field of continence. We have also engaged with a broad group of ward-based nurses and medical staff who are involved in the care of women admitted to hospital.
The study is a collaboration between women, clinicians and researchers, which has underpinned development of the study: need, concept, design and delivery plans. At each stage of the study’s development it has been shared with patients, women of experience and clinicians, the voices of these individuals (and groups) are within all discussions. Our steering group members include women of experience, a geriatrician, a urogynaecologist, a continence nurse specialist, a nursing matron and ward manager, all of whom are in agreement to continue to support development and delivery of the study. Their perspectives and understanding of UI will be drawn on, to enhance clinical understanding of the retrospective quantitative data and the qualitative interview findings.
Ethics and dissemination
The study will be conducted in accordance with the UK Policy Framework for Health and Social Care Research (Health Research Authority (HRA), 2022). Ethical and research governance approvals for the project have been gained through the University of Salford Research Ethics Committee (REC) (project ID: 2972) and HRA (Integrated Research Application System (IRAS) project ID: 303118; REC reference: 21/HRA/4887) through the NHS IRAS. Approval has also been given by the NHS trust collaborating with us to supply anonymised data in the form of statistical outputs (as described in this protocol) to the research team.
The principles of Good Clinical Practice guidelines will be adhered to during recruitment of nurses for the qualitative interviews. Potential participants will be provided with both verbal and written information and given at least 24 hours to decide if they wish to participate. To ensure that the participant’s identity is protected, all personal identifiable information and other potentially identifying (reference to employment or geography) information will be redacted from interview transcripts and final published participant quotes. We will also ensure that participant data are protected in accordance with the Data Protection Act 2018.
Findings will be disseminated through our study website (https://hub.salford.ac.uk/u-inconti/), social media, the press, local NHS clinical meetings and events, and at a range of women’s groups. We will pay particular attention to disseminating outputs to nursing groups such as the Royal College of Nursing (RCN) Bladder and Bowel Society and the general RCN annual conference where we might reach a broad group of nurses able to influence practice and policy. Aside from publishing in open-access peer-reviewed geriatric or urogynaecology journals, we will also present findings at national and international scientific meetings such as the UK Continence Society and International Continence Society where clinical staff and researchers are likely to attend.
Ethics statements
Patient consent for publication
Acknowledgments
Professor Emma Vardy, Jacqueline Rees and Marguerite Heywood.
References
Footnotes
Twitter @hiles_smith, @RuthHaas_HV, @mccarthyjets, @maggieyf
Contributors All authors included made substantial contributions to the conception and/or design of the work, aided in revising and reviewing and have given their final approval of the version to be published. HI-S—conceptualisation, funding acquisition, methodology, supervision, writing (original draft) and writing (review and editing). IMM—conceptualisation, methodology, project administration, writing (original draft) and writing (review and editing). TE-C—conceptualisation, methodology, writing (original draft) and writing (review and editing). LR—conceptualisation and writing (review and editing). REM—conceptualisation and writing (original draft). RHE—conceptualisation and writing (review and editing). JW—conceptualisation and writing (review and editing). MR—conceptualisation and writing (review and editing). LD-H—conceptualisation, methodology and writing (review and editing). YF—conceptualisation, methodology and writing (review and editing). LM—conceptualisation, methodology, writing (review and editing) and supervision.
Funding This work was funded by Health Education England through a development award to the chief investigator.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.



