Article Text
Abstract
Background There is a need to investigate relevant, acceptable and feasible approaches that promote participation in leisure-time physical activity for children with cerebral palsy (CP). The aim of this study is to assess the feasibility of a randomised controlled trial comparing a peer-group intervention focused on improving physical literacy (Sports Stars) with the combination of Sports Stars and a context-focused intervention (Pathways and Resources for Engagement and Participation, PREP) for ambulant children with CP in Brazil.
Methods In this feasibility trial, 18 ambulant children (aged 6–12 years) with CP will be randomised into two groups (nine per group): (1) Sports Stars and (2) Sports Stars plus PREP. The Sports Stars group will receive 8 weekly group sessions, focusing on developing the physical, social, cognitive and psychological skills required to participate in popular Brazilian sports. The combined Sports Stars and PREP group will receive Sports Stars in addition to eight individual PREP sessions focused on overcoming environmental barriers to participation. The primary outcome will include feasibility measures: willingness to participate in an RCT, eligibility and recruitment rates, maintenance of evaluator blinding, acceptability of screening procedures and random allocation, feasibility of evaluating outcomes, contamination between the groups, intervention adherence, treatment satisfaction, understanding of the intervention and implementation resources. Additional instruments will be applied to obtain data related to leisure-time physical activity participation goals, overall participation (home, school and community), physical literacy, level of physical activity and family empowerment. Outcomes will be assessed before, after and 12 weeks after intervention.
Ethics and dissemination This feasibility trial has been approved by ethical Federal University of Minas Gerais’ Ethics Review Committee (CAAE: 33238520.5.0000.5149). All potential subjects will provide written informed consent. The results of this study will be published in peer-reviewed journals and be presented at academic conferences.
Trial registration numbers RBR-4m3b4b6, U1111-1256-4998.
- community child health
- developmental neurology & neurodisability
- paediatric neurology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study strengths include the randomisation and allocation blinding of the evaluator and participants to the intervention groups.
Quantitative and qualitative analyses will provide a comprehensive picture of feasibility.
This study is not powered to show differences and draw conclusions about effectiveness or superiority of the Pathways and Resources for Engagement and Participation or Sports Stars interventions.
Due to the intervention characteristics of this study, it is not possible to blind participants or intervention therapists.
Introduction
Since the publication of the International Classification of Functioning, Disability and Health (ICF) in 2001, participation has been increasingly considered the most important outcome for children and adolescents with disabilities.1 Participation is a complex and multidimensional concept, which includes both attendance (diversity and frequency), and involvement (the experience of being ‘in the moment’). There are also a number of associated participation-related constructs (PRCs) such as activity competence, that influence, but are distinct from, participation.2
Individuals with cerebral palsy (CP) are more likely to experience low levels of participation in leisure-time physical activity participation (ie, sport and physical recreation) than their typically developing peers.3 Participation restrictions in physical activities are found not only due to body structure and function impairments and activity limitations in the physical, cognitive, psychological and social domains of physical literacy4 but also due to contextual barriers at the personal and environmental levels of the ICF. Examples of environmental factors that impact participation include the availability and physical accessibility of sports programmes (environmental), as well as attitudinal factors (personal).5 Interventions aiming to promote leisure-time physical activity participation should address restrictions across these domains.
Leisure-time physical activity participation has the potential to promote well-being and reduce health costs.6 Clutterbuck et al7 proposed the SPORTS Participation Framework (figure 1) to support clinicians’ and researchers’ understanding of the different stages of physical activity participation and to identify appropriate interventions to promote participation in sports and recreation for children with disability. The SPORTS Participation Framework contains six stages that represent the typical pathway that children may progress through as they participate in leisure-time physical activity.7 The first two stages (‘S’ and ‘P’), represent health-focused interventions (individual and group interventions, respectively) that address barriers to participation in sports or physical recreation. For example, building physical literacy skills (eg, gross motor function, team work, confidence and knowledge of sports rules). ‘S’ and ‘P’ stage interventions are important as they facilitate children’s transition to participation in community sports and physical recreation programmes in the following ‘ORTS’ stages, that is, engaging in ‘real-world’ leisure-time physical activity.7
Sports participation model for children with disabilities copied and adapted from Clutterbuck et al.7
Representing the ‘P’ stage, Sports Stars is a practitioner-led, peer-group modified sports intervention that aims to prepare children/adolescents with disability for the transition from usual health-focussed care (eg, individual physical therapy) to leisure-time physical activity participation.8 Sports Stars is designed to target the development of physical literacy, that is, the physical, social, cognitive and psychological skills needed to participate in sport.9 Activities included in the Sports Stars intervention include sports-specific gross motor activity training in a context designed to improve confidence, motivation, teamwork and social skills necessary for ongoing physical activity participation.8 Sports Stars has been investigated in Australia and showed improvements in activity and participation goals in ambulant children with CP.8 According to parents’ and therapists’ perspectives, Sports Stars also improved participants’ overall physical literacy, including physical, social, psychological and cognitive competencies.10
Although Sports Stars has emerged as a promising therapeutic strategy to improve sports participation of individuals with CP, it does not address environmental barriers that might continue to hinder participation in leisure-time physical activity.11 Studies suggest that inadequate community facilities, few availabilities of sports programmes for different age groups, lack of equipment, limited transportation, physical inaccessibility, geographical location, financial constraints, lack of information available for families and attitudinal factors are significant barriers for children with CP to participate in sports and physical recreation.5 Identifying and minimising these barriers, as well as building family support have been shown to be promising intervention strategies to improve the participation of individuals with CP.12 Thus, it is possible that Sports Stars would be more effective if combined with an intervention which addressed these environmental barriers.
Context-focused interventions aiming to produce environmental and behavioural changes have emerged in the last decade.13–15 One example is Pathways and Resources for Engagement and Participation (PREP).15 PREP is a client-centred, individually tailored intervention that aims to promote participation by removing environmental barriers.15 16 Anaby et al15 showed that PREP was effective in improving participation of adolescents with physical disabilities (among them, adolescents with CP) in community-based activities, in addition to body functions and structure and activity (motor, cognition, affective and activity performance) improvements.15 16 PREP has been shown to have a positive impact on family empowerment, likely due to the active involvement of the family during the implementation of the intervention.17
PREP and Sports Stars are both effective in promoting participation in individuals with CP.8 15 However, they do this through different mechanisms. PREP focuses on eliminating environmental barriers that hinder participation in the community15 at the ‘S’ stage of the SPORTS Participation framework, while Sports Stars aims to develop children’s physical literacy8 at the ‘P’ stage. Individual interventions that combine these mechanisms have been shown to be effective for children with CP,18 however, the cost-effective combination of group and individual interventions such as Sports Stars and PREP have not been evaluated. If effective, the implementation of the group-based Sports Stars intervention in conjunction with smaller doses of targeted individual intervention has the potential to reduce health costs at the individual and service level and increase capacity of health services to provide this intervention to a greater number of participants, especially in low-resource settings.
This is particularly relevant in the context of low and middle income countries. In a recent study, Leite et al19 showed that Brazilian children with physical disabilities have low rates of participation (attendance), despite high enjoyment when participating. Despite this, most interventions targeting participation outcomes have not been investigated in low-income and middle-income countries, such as Brazil.3 20 Therefore, it is important that interventions aiming to improve physical activity participation for children with disabilities are evaluated in Brazil, particularly those with the potential to be sustainably upscaled in this context.
The effectiveness of Sports Stars is currently under investigation in a Brazilian context,21 however, the PREP intervention has not been evaluated in Brazil, or for children younger than 12 years old. Therefore, a feasibility study evaluating the combination of these two interventions would be beneficial to investigate if a full clinical trial is warranted and if additional modifications are necessary before performing the full trial. The main aim of this protocol is to assess the feasibility of a randomised controlled trial (RCT) comparing Sports Stars to Sports Stars combined with PREP for ambulant children with CP in Brazil.
Objectives
The objective of this study is to determine the feasibility of conducting an RCT to evaluate the effectiveness of PREP and Sports Stars to improve leisure-time physical activity for children with CP. This includes:
Participants’ willingness to participate in an RCT.
Eligibility and recruitment rates.
Maintenance of evaluator blinding.
Acceptability of screening procedures and random allocation.
Feasibility of evaluating outcomes.
Possible contamination between the groups.
Intervention adherence.
Treatment satisfaction.
Difficulty in understanding the intervention being provided.
Implementation resources.
Methods
Study design
This protocol describes a two-arm, assessor-blinded, RCT. Participants will be randomly allocated to one of two groups: Sports Stars alone or Sports Stars plus PREP. Each group will participate in 8 weeks of intervention. Outcomes will be collected before, after and 12 weeks after the intervention. The study design is illustrated in figure 2.
Study flow chart. COPM, Canadian Occupational Performance Measure; FES, Family Empowerment Scale; GMFCS, Gross Motor Function Classification System; PEM-CY, Participation and Environment Measure for Children and Youth; PREP, Pathways and Resources for Engagement and Participation; PLPQ, Physical Literacy Profile Questionnaire.
Participants and recruitment
Participants will be recruited by advertisement in social media and by convenience from public or philanthropic institutions and private clinics in Belo Horizonte, Brazil. The recruitment process for this study has not started. Children will be eligible if they meet the following inclusion criteria: (1) age 6–12 years old at the beginning of the intervention and (2) diagnosis of CP classified at Gross Motor Function Classification System (GMFCS)22 levels I or II. Children will be excluded if: (1) they have severe cognitive and/or behavioural difficulties that make it impossible to communicate their preferences (as reported by their parents/caregivers); (2) have clinical conditions that prevent them from safely participating in physical activities and (3) have had postoperative orthopaedic and/or neurological surgery in the last 6 months or planned during the study period.
Sample size
This study is designed to investigate the feasibility of conducting a future RCT to evaluate the effectiveness of Sports Stars and Sports Stars plus PREP, and to build decision-making processes to guide the execution of this larger study, particularly concerning recruitment and adherence. Therefore, the sample size was estimated based on the achievement of the primary feasibility outcomes. Secondary treatment effects were not taken into account in calculating sample size as, according to Consolidated Standards of Reporting Trials (CONSORT),23 feasibility studies should not prioritise hypothesis tests to assess the effectiveness or superiority of an intervention.24
The sample size was calculated using the equation below, based on criteria of unacceptable viability (red zone—‘STOP’) vs acceptable feasibility (green zone—‘GO’).25

Where: RUL=upper limit of the red zone; RUL=lower limit of the green zone; Z1−α=probability of type I error; Z1−β=probability of type II error.
In this case, establishing that the adherence rate to the study protocol is ≥65% (green zone), failure rate ≤35% (red zone), alpha of 5% and power of 80%, the sample for the feasibility of the study would be 18 individuals in total, 9 in each group.
In terms of recruitment, experienced clinicians at the trial site have predicted that 50% of eligible patients will agree to participate and complete the research study. Therefore, we plan to identify at least 36 potential participants to reach our target sample (n=18).24 26
Randomisation and blinding
Individual participants will be randomly allocated into one of two intervention groups (group A: Sports Stars or group B: Sports Stars+PREP) by an independent researcher, using a 1:1 allocation ratio.
Block randomisation (block size=18) will be performed using a computer-generated random sequence to ensure equal allocation to each group. Allocations will be concealed in 18 sealed, opaque envelopes numbered 1–18. As this is a group intervention, randomisation will occur at a single time point after enrolment of all participants and completion of baseline assessments. It is therefore unlikely that using the block randomisation method will increase the likelihood of identifying participant allocation.
All randomisation steps will be performed by an independent researcher not involved in recruitment or data collection, and without direct contact with those involved in this research. The independent investigator will oversee the randomisation process and participant allocation. Due to the intervention characteristics of this study, it is not possible to blind participants and intervention therapists to group allocation.
Interventions
Sports stars Brazil
The Sports Stars intervention will be conducted in groups of 6–8 participants. It will be conducted by a physiotherapist with a minimum of 3 years experience working with children with disabilities in a healthcare context and assisted by undergraduate physiotherapy students and physical education professionals. The Sports Stars protocol consists of 8 weekly, 1-hour group intervention sessions. In each session, participants will receive intervention targeting physical literacy competencies relating to participation in popular sports in Brazil: soccer, handball, basketball and athletics. This protocol was based on the original Sports Stars intervention and was previously adapted for Brazilian children with CP.8 21 Participants will receive gross motor activity training related to the reported sports (eg, running, jumping, ball skills) and, will be introduced to the sports in a modified game. The structure and main components of each Sports Stars session are detailed in figure 3. Tasks complexity in each physical literacy domain increases every week based on each child’s level of ability. Standardised descriptors are used to guide this progression as detailed in the sample Sports Stars session plan (online supplemental material 1).
Supplemental material
Structure and main components of the sports stars intervention.
Sports Stars training: All intervention therapists will participate in Sports Stars training with the original creater of the intervention programme. This will include weekly online training over a period of 3 months (approximately 12 hours). Training will focus on the structure of a Sports Stars session, the physical, social, psychological and cognitive content of the Sports Stars intervention, per the Australian Physical Literacy Framework,7 8 and modification and progression of sports activities included in the Sports Stars Brazil session plans. If necessary, the expert who developed the Sports Stars intervention (GC) will be further consulted.
Sports Stars and PREP intervention group
Participants in this group will receive the Sports Stars protocol described above in addition to the PREP protocol. Interventions will be provided simultaneously over the same 8-week period. Both interventions will be conducted by a physiotherapist with a minimum of 3-year experience working with children with disabilities in a healthcare context. In addition to the 8 weekly, 1-hour, group Sports Stars intervention sessions, this group will receive 8 weekly, 1-hour, individual PREP sessions.
PREP sessions will focus on removing environmental barriers to achieve the two goals set at baseline assessment (4 weeks for each goal). Intervention will include involving or coaching the participant and their family to implement solution-based strategies for removing environmental barriers and building on existing supports.15
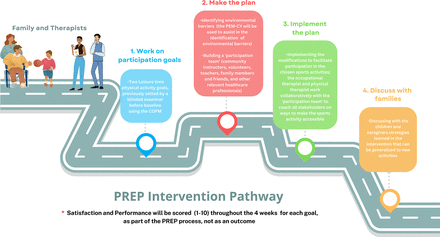
As stated on the manual of the PREP intervention, the Canadian Occupational Performance Measure (COPM) and Participation and Environment Measure for Children and Youth (PEM-CY) are used during the PREP intervention for goal setting and scoring and identification of environmental barriers and facilitators, respectively. As the goal setting/scoring will be performed previously by a blinded assessor at baseline, the full COPM and PEM-CY tools will not be repeated during the PREP intervention. The baseline assessments will be provided to the intervention therapists by an independent assessor to assist the PREP team to make and implement the intervention plan. Children’s goals will be scored in terms of performance and satisfaction during PREP to further guide the intervention. The PREP intervention structure and steps are detailed in figure 4.
Structure and steps of PREP intervention. COPM, Canadian Occupational Performance of Measure; PEM-CY, Participation and Environment Measure - Children and Youth; PREP, Pathways and Resources for Engagement and Participation.
PREP training: PREP intervention therapists will complete the PREP e-learning module available on the Can Child website (https://www.canchild.ca/en/shop/25-prep-intervention-protocol). The PREP manual has been adapted and translated to Brazilian Portuguese by our research group and is now available on the website https://www.prepintervention.ca/. A sample of a PREP intervention Form is provided in online supplemental material 1. If necessary, the expert who developed PREP (DA) will be consulted.
Data collection
Participants characteristics
Personal data and demographic information of children and families will be collected before the study. This will include: (1) motor type (spastic, dyskinetic, ataxic or mixed), (2) distribution (unilateral or bilateral) and (3) gross motor, manual ability and communication classification (GMFCS,22 Manual Ability Classification System27 and Communication Function Classification System).28
Measures
Primary and secondary outcome measures will be performed at three time points: baseline, immediately postintervention and 12-week follow-up.
Primary outcome measure
Feasibility
This protocol was designed to analyse the feasibility of combining Sports Stars and PREP with the aim of improving participation in leisure-time physical activity for ambulant children with CP. The definition of a feasibility study encompasses the question: ‘Can this study be done?’. The Standard Protocol Items for Randomised Interventional Trials29 and the CONSORT statement extension to pilot and feasibility randomised trials were followed in the planning of the study and reporting of the protocol. The results will later be reported following the CONSORT guidelines.30
Feasibility measures will include (1) willingness to participate in an RCT, (2) eligibility and recruitment rates, (3) feasibility of assessor blinding, (4) acceptability of screening procedures and random allocation, (5) possible contamination between the groups, (6) feasibility of evaluating outcomes (7) intervention adherence, (8) treatment satisfaction, (9) difficulty in understanding the intervention being provided and (10) implementation resources. All feasibility measures were adapted from studies by Sharma et al31 and Feitosa et al.32 Questionnaires will be completed by participants’ parents/caregivers postintervention and are described in detail in online supplemental material 2.
Supplemental material
Criteria for feasibility
The results of this feasibility trial will identify if the study as presented is feasible, which will guide recommendations for a full trial to evaluate intervention effectiveness. The decision will be one of the following: (1) do not continue to a full trial if any preplanned changes may not help improve the feasibility; (2) modify the design further before conducting a full trial; (3) continue with the full trial applying the same procedures used in the feasibility trial with no changes; however, include close monitoring to ensure that study procedures are closely followed and (4) continue with the full trial applying the same procedures used in the feasibility trial with no changes. Close monitoring is not necessary.31 33 The full criteria for the outcome of feasibility are presented in table 1. Given the nature of some feasibility measures (acceptability of random allocation, acceptability of screening procedures, treatment satisfaction, difficulty in understanding the intervention being provided and implementation resources), they will not have cut-off criteria to determine the feasibility of performing a complete RCT and are therefore not presented in table 1. Instead, their results will be reported descriptively and will be used to determine the suitability of a full RCT combined with the others specific criteria.
Criteria for feasibility
Additional measures
Additional measures will be administered to obtain data related to (1) leisure-time physical activity participation goals; (2) overall participation at home, school and community; (3) level of physical activity; (4) physical literacy and (5) family empowerment. These measures are included primarily to evaluate their feasibility for a future RCT, not to evaluate the effectiveness of the interventions.
Canadian Occupational Performance Measure
The COPM is a frequently used measure of individual, client-centred outcomes which focuses on the goals and priorities of the child and family in paediatric rehabilitation.34 A modified COPM approach has been used previously to set goals in specific areas, for example, targeting activity or participation outcomes.8 18 35 The standard COPM includes discussion about self-care, productivity and leisure. In this study, a modified COPM approach will be used to target the leisure domain, specificially leisure-time physical activity participation.
Children in both groups will participate in goal setting at baseline (before randomisation) to identify two important, relevant and meaningful leisure-time physical activity participation goals that they would like to work towards regardless of intervention. These goals must relate to leisure-time physical activity (ie, sport or physical recreation), and must be involve participation per the ICF (ie, involvement in a life situation). Goals will be classified as attendance goals (ie, goals relating to the diversity, frequency or duration of participation), or involvement goals (ie, goals relating to being ‘in the moment’ during attendance), per the family of PRC.2 During goal setting, families will be supported to reframe goals not fitting the criteria of (1) focusing on leisure-time physical activity or (2) targeting participation (ie, attendance or involvement).
To develop and rate these goals, parents or caregivers of children will complete the modified full COPM interview with a blinded assessor. After identifying the two leisure-time physical activity participation goals, parents/caregivers will rate their perception of children’s performance and satisfaction on a 10-point scale. This rating process will be repeated post-treatment, and at 12-week follow-up for children in both groups. The COPM is reliable and valid36 and can detect changes in performance over time and after an intervention.34 A change in score of two points or more is considered clinically significant.36
Children’s leisure-time physical activity goals will be provided to the PREP team by an independent assessor to ensure consistency in intervention focus, and prevent confusion, which may occur if the COPM was repeated during the PREP intervention. Performance and satisfaction of the established goals will be rated during the PREP intervention per established protocols.15
Participation and Environment Measure for Children and Youth
The PEM-CY is an outcome measure generated by parents' perceptions, which evaluates components of participation and environment for children and adolescents with disabilities in three different settings: home, school and community.37 For this study, the school (5 items) and community (10 items) sections will be used. Parents will indicate the frequency of participation of their children (8-point scale from daily=7 to never=0), typical involvement during participation (5-point scale from 1 being minimally involved to 5 being very involved), as well as if they wish to see a change in the frequency of participation and/or involvement of the child (yes/no and five options for the type of change desired). The PEM-CY also assesses the extent to which environmental factors, support and resources in each setting are barriers and/or facilitators (16 items for the community and 17 items for school). Mean scores for participation frequency and involvement will be calculated. The PEM-CY has been adapted and validated in the Brazilian context.38
The PEM-CY collected at baseline will also be used to guide the PREP intervention. For children allocated to the Sports Stars plus PREP group, an independent assessor will pass the baseline PEM-CY information to PREP intervention therapist, in order to assist in identifying barriers and facilitators during the PREP intervention.
Physical activity levels
Physical activity levels will be evaluated using free-living, tri-axial accelerometry, a valid and reliable method used for measuring habitual physical activity in children with CP.39 The Actigraph wGT3X-BT (ActiGraph, LLC, Pensacola, Florida, USA will be the model used. This accelerometer provides the magnitude of trunk acceleration in three planes at a set frequency of 30 Hz. At baseline, post-treatment and follow-up, the ActiGraph device will be placed on the hip of each child (secured around the waist above the iliac spines on the dominant side) and worn for seven consecutive days (five week days and two weekend days). The device will be used during usual activities of daily living in home, school and community environments; and removed during sleep and water activities. Parents or caregivers will record the device’s on and off times in a daily log. Devices will be returned after day 7 for data extraction. Periods of non-wear will be automatically detected by the device.39
The following data will be analysed through the ActiLife V.6 software (ActiGraph, Pensacola, Florida, USA): total time spent in light physical activity (LPA), moderate-to-vigorous physical activity (MVPA) and sedentary behaviour. The cut-off points described by Baque et al40 will be used in this study: sedentary time (0–100 counts/15 s), LPA (101–468 counts/15 s) and MVPA (≥469 counts/15 s).40
Physical Literacy Profile Questionnaire
Physical literacy will be assessed using the Physical Literacy Profile Questionnaire (PLPQ). This instrument was developed using the Australian Physical Literacy Framework definition of physical literacy9 to provide a proxy report of children’s physical, cognitive, social and psychological performance in the context of physical activity participation. The PLPQ includes 24 items related to these four physical literacy domains. Parents will be asked to rate each item based on their child’s competence. On a three point scale (0) does not perform, (1) performs partially and (2) performs completely). The maximum score is 48 points, which is converted to a percentage score. This instrument is under the process of analysis of its measurement properties by this research group. Preliminary data from our ongoing study with children, and young people with disabilities (6–21 years old, most children with CP at all GMFCS levels) showed that the PLPQ has appropriate face validity, good internal consistency and test–retest reliability for children with CP (α=0.93, ICC=0.86; 95% CI: 0.75 to 0.92) (data not yet published).
Family Empowerment Scale
The Family Empowerment Scale (FES) is a self-administered questionnaire that was developed to measure the empowerment status of the family of parents whose children have some type of disability.41 FES assesses four levels of empowerment: (A) militancy system, which assesses parents’ values and beliefs in the light of public policy and services offered; (B) knowledge, which assesses parents’ knowledge, as well as their relationship with the professionals involved in the care of their children; (C) competence, which verifies the parents’ ability to solve problems and (D) self-efficacy, which assesses the parents’ perception of the care proposals offered by health professionals. The FES is a 5-point Likert scale (‘never’, ‘rarely’, ‘sometimes’, ‘often’ or ‘very often’), with a total of 34 items. FES is a reliable and valid measurement as demonstrated by previous studies.42 43 It has been translated to Brazilian Portuguese and its reliability is under investigation by our research group.
Data analysis
To assess feasibility, a descriptive data analysis will be implemented. The analysis plans for the main feasibility objectives are described in the feasibility item of this section. The baseline clinical and demographic characteristics of participants in both arms of the study will be compared descriptively. Descriptive statistics (mean and SD), and the proportion of participants who completed each measure, will be reported for each outcome at baseline, immediately and 12 weeks post-intervention. This will provide data to assist in determining the most appropriate outcome for a future trial.
Treatment effects for secondary outcome measures will be presented as means, SD and CIs. As this is a feasibility study, no statistical significance tests or hypotheses regarding the effectiveness of the treatment will be performed. The analysis will be based on intention to treat and will be exploratory. Cohen’s criteria will be followed to value the effect sizes of the studied variables, though due to the pilot nature of the study, all the effect analyses must be considered exploratory only.44 Cohen’s d will be obtained by dividing effect sizes by pooled baseline SD and the following thresholds will be considered for interpretation of effect size: small (0.20–0.49), medium (0.50–0.79) and large (>0.80).44 High scores indicate better outcomes and positive effect sizes suggest benefit from Sports Stars and PREP interventions. Minimal clinically important difference will be examined for outcomes when available. The number (and per cent) of participants in each group achieving clinically significant change will be explored descriptively. Based on this analysis, an appropriate primary outcome will be identified and will be used to estimate the sample size for a future RCT.
No imputation methods will be used to handle missing data, rather missing cases will be excluded from analysis. Analysis of covariance will be used to estimate effect size for each outcome variable immediately and 12 weeks post-intervention follow-up, adjusting for their baseline values. Statistical analyses will be conducted using IBM SPSS Statistics for Windows (V.22.0, IBM).
Patient and public involvement
Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. More details have been provided throughout this section.
Ethics and dissemination
This study will be assessor-blinded, two-arm, feasibility RCT and has been prospectively registered at Brazilian Clinical Trials (Registry: RBR-4m3b4b6). Full ethical approval has been obtained from the Federal University of Minas Gerais’ Ethics Review Committee (CAAE: 33238520.5.0000.5149). Written consent will be obtained from parents or caregivers of each participant. Written assent will be obtained from each child. Participants’ information will be coded to preserve their identity.
On completion of the study, data will be analysed and tabulated and a final study report will be prepared. Members of the research team will write the final articles. Inclusion and order of authorship will be guided by contribution levels. Study results will be published in peer-reviewed academic journals, as well as presented at national and international conferences. Study results will be shared with participants using a lay summary.
Discussion
This study presents a clinical trial protocol aimed at evaluating the feasibility of a definitive controlled trial comparing Sports Stars versus Sports Stars plus PREP for ambulant children with CP in Brazil. In addition, it aims to explore outcomes such as participation, physical literacy, level of physical activity and family empowerment for a future trial. The findings of this study will inform the development of a future RCT to investigate the effectiveness and superiority of Sports Stars Brazil compared with Sports Stars plus PREP. This feasibility study will identify if modifications are required to the protocol prior to undertaking a full trial.
Limitations of this study include the absence of outcome measures with strong psychometric evidence for children with CP. The COPM is the only outcome with reported minimum clinically important difference (MCID). While the PEM-CY has been shown to be an important part of the PREP protocol, it has not shown strong evidence of responsiveness to intervention. Regarding the measurement of physical literacy, the PLPQ questionnaire is a parent-reported measure, and no other objective measure is available to evaluate this outcome. Finally, this feasibility study is not powered to detect a significant difference in outcome measures, however, is an important step towards designing a comprehensive RCT to test the efficacy of intervention programmes designed to increase participation of children with CP.
This study is an important step in evaluating pragmatic interventions aiming to improve leisure-time physical activity participation for children with disability in low-income and middle-income countries 45. The data of the full clinical trial will have potential clinical implications for the rehabilitation scenario in Brazil, similar cultures and other low-income and middle-income countries, giving empirical evidence about the combination of Sports Stars and PREP as a feasible and potentially effective intervention for promoting leisure-time physical activity participationfor children with CP.
Ethics statements
Patient consent for publication
Acknowledgments
We thank the Universidade Federal de Minas Gerais (UFMG) and the Pró Reitoria de Pesquisa da UFMG for their institutional support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @no, @GeorginaPaedsPT, @LongoEgmar, @herculesleite5
Contributors DOS, LCdS, RRdSJ, GC, DA, EL, RCM, ACRC and HRL were responsible for the study concept, design and ethics applications. RRdSJ registered the trial with the Brazilian Register of Clinical Trials-REBEC and DOS drafted the manuscript which was reviewed by the authors. All authors read and approved the final manuscript.
Funding This work was supported by CNPq (Process 150010/2022-2), CAPES (Proex, PROCESS - 23038.002128/2022-08) and FAPEMIG (APQ-00754-20).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.