Article Text
Abstract
Introduction Substance use disorders (SUDs) take an enormous toll on US Veterans and civilians alike. Existing empirically supported interventions vary by substance and demonstrate only moderate efficacy. Non-invasive brain stimulation represents an innovative treatment for SUDs, yet aspects of traditional neurostimulation may hinder its implementation in SUD populations. Synchronised transcranial magnetic stimulation (sTMS) uses rotating rare earth magnets to deliver low-field stimulation synchronised to an individual’s alpha peak frequency that is safe for at-home administration. The current trial aims to assess the acceptability and feasibility of sTMS, as well as the safety of at-home sTMS administration for substance-disordered Veterans.
Methods and analysis Sixty Veterans in substance treatment at the Providence Veterans Affairs will be randomised to receive 6 weeks of active or sham sTMS treatment. Eligibility will be confirmed by meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for an alcohol, cocaine or opioid use disorder. Daily supervised sTMS treatment will occur either in clinic or at home through video monitoring. Clinical and self-report assessments will be completed at baseline, end of treatment and 1-month follow-up. Urine drug screening will occur once per week during the treatment phase. Primary outcomes include treatment adherence/retention and satisfaction to evaluate sTMS feasibility and acceptability in Veterans with SUDs. The safety of at-home sTMS administration will be assessed via adverse event monitoring.
Ethics and dissemination The sTMS device received a significant risk determination for at-home use by the Food and Drug Administration in July 2021. Ethics approval was obtained in August 2021 from the Providence Veterans Affairs institutional review board and research and development committee. Data collection began in September 2021 and is planned to continue through December 2023. Findings will be disseminated at national conferences and in peer-reviewed journals. Results will serve to inform the development of large-scale clinical trials of sTMS efficacy for substance-disordered Veterans.
Trial registration number ClinicalTrials.gov Registry (NCT04336293).
- Telemedicine
- MENTAL HEALTH
- Substance misuse
- Clinical trials
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Synchronised transcranial magnetic stimulation (sTMS) is a novel form of neuromodulation that has yet to be investigated for the treatment of substance use disorders (SUDs).
This protocol implements a double-blind randomised sham-control design to evaluate the acceptability, feasibility and safety of sTMS in Veterans with alcohol, cocaine or opioid use disorders.
This trial will measure the safety of at-home sTMS administration, and thus lay the foundation for future efficacy trials for a portable, patient-operated, neurostimulation treatment for SUDs.
Enrolment will be limited to 60 Veterans (20 participants each with alcohol, cocaine and opioid use disorder, respectively) and will therefore not produce a sample large enough to evaluate sTMS efficacy for substance-related outcomes.
Participants will not be randomised to at-home or in-clinic treatment administration, which creates the potential for patient self-selection biases and impairs active versus sham treatment balancing across the treatment delivery locations.
Introduction
Substance use disorders (SUDs) disproportionately affect US Veterans, with treatment costs exceeding $350 million annually within the Veterans Health Administration alone.1 2 However, empirically supported pharmacological and behavioural treatments vary by substance and display only moderate efficacy.3–5 Therefore, alternative SUD treatments, such as non-invasive neurostimulation, warrant investigation.
Trials investigating the effect of the most common form of neurostimulation, repetitive transcranial magnetic stimulation (rTMS), in reducing substance-specific cravings have produced varying degrees of success for those with alcohol, cocaine or opioid use disorders.6–10 Mixed findings may be due to the nature of rTMS and how the device is calibrated for treatment.11 12 Standard rTMS involves device calibration to individual cortical excitability, yet precisely how substance use changes cortical excitability remains unclear.13 Any such changes to neural reactivity in substance users could increase the risk of seizure through the application of too much energy.14 Conversely, treatment non-response is possible if too little energy is delivered. To optimise the likelihood of treatment success, and increase safety for those with SUDs, the development of an intervention that can provide low-level stimulation and enhance access though at-home use is critical. These concerns highlight synchronised TMS (sTMS), which delivers non-invasive magnetic energy calibrated to a person’s individualised alpha frequency (IAF) measured via electroencephalography (EEG), as a novel SUD treatment alternative.15
Furthermore, spatial targeting within neurostimulation for SUDs continues to be heavily debated.16 17 In a review of TMS for the treatment of depression, Philip et al18 found a lack of consensus regarding target site parameters, thus raising the question of whether precise spatial targeting is necessary for treatment success.18 sTMS operates through the application of energy to midline brain regions more broadly and has received preliminary support in the treatment of depression and post-traumatic stress disorder (PTSD), reinforcing the notion that spatial targeting may not be essential.19 20 The building evidence that TMS effects are not brain region specific opens the door to research accounting for frequency specificity, such as stimulation calibrated to an individualised frequency.
Treatment retention is another challenge for empirically supported SUD treatments.21 Compared with traditional rTMS, which involves daily outpatient appointments over the course of many weeks, the sTMS device, manufactured by Wave Neuroscience, can be operated by patients in their homes.22 An investigation of the safety of at-home sTMS for SUDs could reduce burden among a clinical population that faces tremendous barriers to treatment success.23 In sum, the factors listed above imply that sTMS may serve as a novel treatment for Veterans with SUDs.
Current aims
Our primary objective is to conduct the first study to deliver sTMS to Veterans with alcohol, cocaine or opioid use disorders. Two specific aims will be addressed. First, this study serves to assess the acceptability and feasibility of sTMS among Veterans with SUDs using the Wave Neuroscience device in a pilot sham-controlled trial. Second, we will evaluate the safety of in-laboratory and at-home sTMS administration for substance-disordered Veterans. Our hope is to lay the groundwork for larger-scale clinical trials that will evaluate the efficacy of sTMS to help those with addiction, particularly through the establishment of at-home neurostimulation treatment.
Methods and analysis
Sample size calculation
At least 20 subjects will be enrolled for each of the three substances focused on in this study (alcohol, cocaine, opioids) for a total N of 60. A previous study focused on different sTMS parameters for SUD used a sample size of ~N=20.24 The sample size for this pilot-controlled study is based on estimations focused on the amount of information required to inform next steps in trial design, rather than on statistically significant calculations for a primary safety or efficacy endpoint. By employing a comparable sample size for each substance, we anticipate having sufficient power to detect significant differences between baseline and endpoint. This sample size is adequate to determine the appropriate sample size for subsequent trials.
Participants
Individuals will be eligible to participate if they (1) are Veterans affiliated with Veterans Affairs (VA) Providence, Providence, Rhode Island; (2) meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria for SUD; and (3) are 18–70 years of age (inclusive) (see table 1 for full list of inclusion criteria).
Participant inclusion and exclusion criteria for study
Participants will be excluded if they (1) have greater than a mild traumatic brain injury; (2) have a current or significant past neurological disorder including seizure, primary or secondary central nervous system tremor, stroke or cerebral aneurysm; (3) have a severe psychiatric disorder that requires immediate clinical attention (eg, psychosis, suicidal ideation with intent and plan); and/or (4) have implanted devices activated or controlled by physiological signals (eg, cardiac pacemakers, implanted medication pumps, etc). Participants must also (5) not have an implanted device or metal in the brain, cervical spinal cord or upper thoracic spinal cord (see table 1 for full list of exclusion criteria).
Procedures
Recruitment and screening
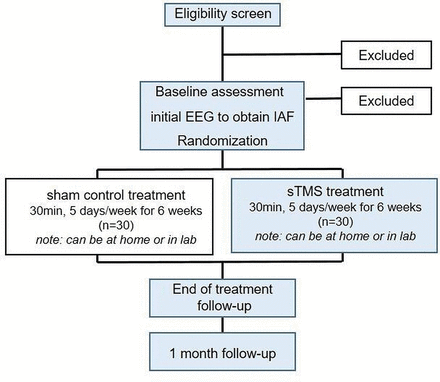
Up to 60 participants who meet criteria for a DSM-V SUD will be recruited to complete this study (see figure 1), with 20 identifying alcohol as their drug of choice, 20 identifying cocaine as their drug of choice and 20 identifying opiates as their drug of choice. Veterans will be recruited through the Collaborative Addiction and Recovery Services Programme at VA Providence. Potential participants may call in response to advertisements for the study or will be referred by clinicians (by giving patients the study ad). Research assistants will assess preliminary demographic eligibility criteria upon phone screen. Those who meet preliminary eligibility criteria will be invited to the laboratory for a baseline visit. After providing written informed consent, psychiatric interview and self-report measures will be used to confirm eligibility with regard to diagnosis, past psychotropic treatment, current health and current symptom severity (see table 2 for full list of assessments). There is a requirement for maintenance on a stable regimen of psychotropic medications (if applicable) for 6 weeks prior to baseline and during participation in sTMS treatment.
Participant flow diagram (n=60). This includes individuals from all three substance groups (alcohol, cocaine and opioids). EEG, electroencephalogram; IAF, individualised alpha frequency; sTMS, synchronised transcranial magnetic stimulation.
Measures by time point assessed and mode of administration
Baseline
Participants meeting initial phone screen eligibility will be invited to a baseline visit. Research staff will review and obtain written informed consent for either at-home administration or in-laboratory administration prior to the initiation of study procedures.
Psychiatric interviews and self-report measures will confirm eligibility regarding SUD diagnosis, past psychiatric treatment, current health and current symptom severity. Demographic and clinical data include gender, age, substance use (quantity and frequency) and comorbid symptomatology, which will be collected to quantify change due to sTMS, or as factors that may influence the effect and tolerability of sTMS. Participants will complete a timeline follow-back (TLFB) to confirm substance use over the past 30 days.25 The Quick Structured Clinical Interview for DSM-5 Disorders26 will assess diagnostic criteria for alcohol, cocaine or opioid use disorder.
Laboratory assessments will quantify substance use and include a urine drug screen, ethyl glucuronide test and the following liver function tests: gamma-glutamyl transferase (GGT; µ/L), serum glutamic-oxaloacetic transaminase/aspartate aminotransferase (SGOT/AST; µ/L), serum glutamic pyruvic aminotransferase/alanine aminotransferase (SGPT/ALT; µ/L), total bilirubin (mg/dL). Prior research shows that response to neurostimulation may be related to genetic or epigenetic differences between people. Accordingly, an additional blood sample will be collected, with DNA extracted and assessed using genome-wide and epigenome-wide analyses to investigate genetic and epigenetic differences in the context of treatment response.
Drug/alcohol cue reactivity will be measured via a task presented using E-Prime 3 software (Psychology Software Tools, 2017)27 following work by the Hanlon group in the area of cue reactivity and TMS. The computer task consists of six 96-second blocks. The first three blocks contain neutral images (eg, glass of water, cooking utensils, people eating dinner). The last three blocks contain images of drug-related or alcohol-related stimuli customised for each group (eg, crack pipe for cocaine users, liquor bottles for alcohol users). Prior to starting the task and at the end of each block, participants will record their substance cravings on a paper assessment.
Participants will undergo a 10-minute resting state EEG after which de-identified data will be shared with the sTMS device manufacturer, Wave Neuroscience, for analyses. These analyses will capture participants’ IAF, a marker of interindividual differences in EEG rhythms, which will indicate the optimal magnetic field frequency for treatment.
Randomisation and blinding
Participants will be randomised into either active sTMS or sham sTMS treatment groups. Treatment will be delivered in a double-blind fashion so that neither participants nor research staff will be aware of study condition. The sham sTMS device has an external appearance, weight, sound and operation indistinguishable from the active sTMS device with a non-magnetic rotating metal shaft replacing the rotating neodymium magnets to reduce the potential for unblinding. Participants will self-select into either at-home or in-laboratory sTMS administration procedures.
Treatment phase
sTMS device
The study will use the Wave Neuroscience sTMS device, which consists of three main elements: (1) headset, (2) patient passport module (PPM) and (3) base station. The PPM is a USB flash drive containing an encrypted file with the IAF device parameter as well as a code to specify whether the PPM is destined for an active or sham device. If an active PPM is inserted into a sham device or vice versa, the display on the base station will show ‘invalid PPM’.
At-home administration
Treatment sessions will be completed in participants’ homes using a portable sTMS device. Acting under the supervision of a TMS-credentialed physician, trained research staff will observe all 30 in-home treatments (five per week) through video technology to ensure that participants are awake and using the device correctly. Treatment emergent side effects associated with stimulation (during treatments) and emerging between treatment sessions will be queried on each treatment day and recorded into participants’ medical charts.
In-laboratory administration
Trained research staff will be present for all sTMS sessions at VA Providence. During the sTMS session, study staff will ensure that participants are awake and using the device correctly. Treatment emergent side effects will be queried and recorded on each treatment day. Appropriate medical coverage is available at all times.
Common treatment procedures
sTMS will be delivered following Wave Neuroscience guidelines using the device user manual. Each participant’s IAF will be displayed on the device LCD screen once the PPM is plugged in. Before initiating treatment sessions, study staff will confirm that the IAF parameters displayed on the device LCD screen match the IAF provided by Wave Neuroscience. If the values do not match, treatment will not be administered. Participants will be instructed to remove jewellery above their shoulders and anything from their mouth (eg, gum) that could generate facial muscle activity. They will then secure the sTMS device to their heads, lie down in a semireclined position and turn the magnetic adjustment knobs. After pressing the start button, the device will rotate the magnets for 30 min, at which point rotation ceases and the session ends. Sessions may be paused or cancelled at any time; however, once cancelled, or completed, the device is programmed such that a new session cannot be started for 10 hours. This prevents subjects from excessively using the device while not under the direct supervision of study staff.
Weekly in-person visits will occur across the treatment phase (six weekly visits total). Participants will complete a TLFB, brief self-reports and provide a urine drug screen. Additional measures will be taken by research staff to protect against COVID-19 infection including pre-appointment COVID-19 screening, personal protective equipment, etc.
Follow-up assessments
Two post-treatment appointments will occur: an end of treatment (EOT) visit 72 hours after the final treatment session, and a 1-month follow-up. At both post-treatment visits, participants will complete self-report questionnaires, the drug/cue reactivity task, a TLFB and a urine drug screen. At the EOT visit, participants will additionally be asked to complete a treatment satisfaction questionnaire and condition blinding questionnaire to ensure they were blinded to study condition.
Compensation
Participants will be offered compensation for completion of specific milestones in the study: $50 for completion of all baseline procedures, $100 upon completion of all 30 sTMS treatments, plus another $75 for completing the 1-month follow-up, totalling $225. Payment will be offered in the form of gift cards or electronic fund transfer.
Primary outcomes
Aim 1: to demonstrate feasibility and acceptability of at-home and in-laboratory sTMS among Veterans with specific SUDs (ie, alcohol, cocaine, opioids) in a pilot sham-controlled study. Thirty sTMS treatment sessions will occur with trained research staff monitoring in person or through video technology to verify that participants are awake and using the device properly. Feasibility will be evaluated by rates of recruitment, treatment adherence, retention and completion of assessments (see table 3). Acceptability will be measured by retention and participant reports of acceptability and satisfaction (see table 3).
Primary clinical and mechanistic outcomes
Although we anticipate the sample will not be large enough to provide adequate statistical power to test for differences between sTMS and sham stimulation, we anticipate collecting feasibility data and will generate CIs around all observed effect sizes.
Aim 2: to evaluate the safety of in-laboratory and at-home sTMS among Veterans with SUDs. Safety of at-home sTMS administration for Veterans with alcohol, cocaine and opioid use disorder will be assessed through adverse event monitoring. Daily safety questions will probe potential treatment-related side effects and changes in substance use. Medications will be monitored through self-report and electronic medical record review. Adverse events will immediately be reported to the principal investigator (PI).
Other outcomes
The clinical interview assessment of substance use symptoms will include the Clinical Global Impressions–Severity (CGI-S)28 to quantify the severity of the participant’s mental illness at the time of assessment; Social Occupational Functioning Assessment Scale29 to quantify the participant’s level of social functioning in daily life at the time of assessment; and CGI–Improvement (CGI-I) assessment to quantify the level of improvement in participants’ illness from baseline to the time of assessment.
The following self-report questionnaires will be administered in order to quantify measures of PTSD, depression, quality of life, affect and sleep to assess how these constructs may be related to substance use, craving and sTMS treatment feasibility: Clinician-Administered PTSD Scale,30 PTSD Checklist for DSM-V,31 the Life Events Checklist,32 Inventory of Depression Symptomatology Self-Report,33 34 Positive and Negative Affect Schedule,35 State-Trait Anxiety Inventory,36 Quality of Life Enjoyment and Satisfaction Questionnaire,37 Pittsburgh Sleep Quality Index,38 Adaptation Satisfaction with Treatment Form and Treatment Blinding Questionnaire.
Data analysis plan
Data management and confidentiality
Only research staff who have undergone the relevant responsible research conduct and handling of private and confidential information training will handle study data. These data will only be used for research purposes. A unique identification number for each participant will be used on all assessments in lieu of any identifying information. Additionally, analyses will be completed on de-identified data.
Missing data (ie, participants lost to follow-up) will be handled using full information maximum likelihood estimation for statistical models in our primary analyses. This type of approach can easily be implemented in model-based software packages, such as MPlus. Moreover, all available cases will contribute to the computation of the maximum likelihood estimates, providing the most likely results based on the observed data. Additionally, exploratory and sensitivity analyses will be conducted to characterise patterns of missingness and determine whether systematic similarities exist for participants who were lost to follow-up.
Aim 1: feasibility and acceptability
Adequate feasibility of the intervention will be indicated by a recruitment rate of two or more patients per month and retention rates of 50% or higher completed assessments based on previous sTMS trials19 and empirical evidence from 3-month treatment programmes.39 Additionally, acceptable rates of treatment adherence will be completion of at least 80% of the treatment sessions as defined by a previous study that showed an effect of sTMS on depression.18
Aim 2: safety of in-laboratory and at-home sTMS
In order to evaluate safety, we will meticulously monitor all adverse events that occur during the study. Adverse events will be captured using a combination of clinician interviews and spontaneous adverse event reports (coded using the current version of the Medical Dictionary for Regulatory Activities), and through systematic self-report using the Systematic Assessment for Treatment Emergent Effects (SAFTEE).40 All adverse events will be assessed and described in terms of the relationship to the device, relationship to the procedure, severity of the event, subsequent treatment or intervention, and the resolution status. Medications will be followed at each study visit and corroborated with the VA electronic medical record. Any adverse events that occur while participants are using the sTMS device at home will be captured by trained research staff who will observe all 30 at-home treatment sessions via video technology. All reported adverse events will be logged and reported to the PI.
Patient and public involvement
As part of this pilot trial, participants will provide important feedback on feasibility and safety through communication with research staff, as well as a treatment satisfaction questionnaire that assesses the burden of the intervention. Participants and members of the public were not involved in the design of study procedures. We will use feedback to inform efficacy trials.
Ethics and dissemination
Ethics
All study procedures were approved by the Providence VA institutional review board (IRB) and research and development committee. Serious and unexpected adverse events will be reported to the IRB within 24 hours while potential adverse events will be reported during annual continuing reviews. The sTMS device has received a significant risk determination for at-home use by the Food and Drug Administration. As such, an independent data safety monitoring board composed of individuals not affiliated with the study will convene on at least a quarterly basis to review all relevant data pertaining to participant safety.
To address the risk of worsening SUD symptoms, substance use will be monitored with prescribed cut-offs in substance use assessments acting as indicators that symptoms may be worsening. Participants deemed at risk will be withdrawn and referred to the Providence VA Collaborative Addiction and Recovery Services Clinic. Participants endorsing significant withdrawal symptoms will be instructed to seek immediate medical treatment. The PI will discontinue the trial if (1) participants experience any serious adverse events found to be attributable to sTMS; (2) two participants experience clinically meaningful deterioration in suicidal ideation or (3) any participant attempts suicide.
Dissemination
This study will lay the groundwork for large-scale clinical trials that will evaluate the efficacy of sTMS as a treatment for SUD. The results of this pilot sham-controlled trial will be disseminated to maximise the impact of preliminary findings. The PI will share de-identified datasets, statistics and results collected from this proposal by depositing these data at the National Library of Medicine PubMed Central website repository as this is a VA-supported data repository. Planned manuscripts include a primary outcome paper(s) describing sTMS treatment feasibility for Veterans with substance use disorders (ie, alcohol, cocaine, opioids). Results of this study will be presented at national conferences such as Research Society on Alcoholism and College on Problems of Drug Dependence.
Ethics statements
Patient consent for publication
Acknowledgments
We thank research participants and our clinical collaborators at the Providence VA Medical Center for their ongoing support.
References
Footnotes
JJ and MJQ are joint first authors.
Contributors JJ and JEM drafted the initial proposal, with input from NSP, RMS and LB. MJQ, JLC and CBB-B drafted the manuscript, which all authors reviewed and revised.
Funding This work was supported by the Center for Neurorestoration and Neurotechnology (N2864-C) and RR&D Small Projects in Rehabilitation Research (SPiRE; 5I21RX003338) from the US Department of Veterans Affairs, Rehabilitation Research and Development Service, Providence, Rhode Island, USA. Device support on this project is supported through clinical trial contracts between Wave Neuroscience and the Ocean State Research Institute and Veterans Affairs Providence.
Disclaimer The contents do not represent the views of the US Department of Veterans Affairs or the US Government. Neither the funders nor Wave Neuroscience was involved in the decision to conduct the study or study procedures.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.