Article Text
Abstract
Introduction Advances in medical technology and postoperative care have led to increased survival of children with medical complexity (CMC). Parents of CMC develop substantial caregiver expertise and familiarity with paediatric intensive care unit (PICU) staff and treatment procedures which may give rise to tensions regarding respective roles, caretaking preferences, treatment goals and expected outcomes. A therapeutic alliance built through strong partnerships constitutes the foundation of patient and family-centred care (PFCC), contributing to improvements in experiences and outcomes. Yet acute care settings continue to struggle with integrating PFCC into practice. This study aims to enhance PFCC for CMC in the PICU using an innovative approach to integrated knowledge translation.
Methods A mixed-method concurrent triangulation design will be used to develop, implement and evaluate PFCC practice changes for CMC in the PICU. Qualitative data will be collected using an Experience-based Co-design (EBCD) approach. Parents, CMC and staff will reflect on their PICU care experiences (stages 1 and 2), identify priorities for improvement (stage 3), devise strategies to implement changes (stage 4), evaluate practice changes and study process, and disseminate findings (stage 5). The quantitative arm will consist of a prepractice and postpractice change evaluation, compared with a control site. Analysis of qualitative and quantitative data will provide insights regarding the impact of PICU practice changes on PFCC.
Ethics and dissemination The McGill University Health Centre Research Ethics Board (Ref. #2019-5021) and the Hospital for Sick Children Research Ethics Board (Ref. #1000063801) approved the study. Knowledge users and researchers will be engaged as partners throughout the study as per our participatory approach. Knowledge products will include a short film featuring themes and video/audio clips from the interviews, recommendations for improvements in care, and presentations for healthcare leaders and clinical teams, in addition to traditional academic outputs such as conference presentations and publications.
- Paediatric intensive & critical care
- MEDICAL EDUCATION & TRAINING
- Paediatric intensive & critical care
- EDUCATION & TRAINING (see Medical Education & Training)
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Paediatric intensive & critical care
- MEDICAL EDUCATION & TRAINING
- Paediatric intensive & critical care
- EDUCATION & TRAINING (see Medical Education & Training)
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study aims to enhance patient and family-centred care for children with medical complexity in the paediatric intensive care unit (PICU) using Experience-based Co-design (EBCD), an innovative approach to integrated knowledge translation.
This will be the first study to use EBCD to improve patient and family-centred care experiences for children with medical complexity, parents and healthcare professionals in the PICU.
Knowledge users (children, parents, healthcare professionals) will be engaged as partners to identify, codesign and implement practice changes to enhance patient and family-centred care for children with medical complexity, a PICU population that staff may be less familiar with through training, experience or expertise.
Findings will inform healthcare professional training and medical/health sciences education regarding this growing PICU population.
Implementing practice changes in the PICU environment may present challenges; however, to optimise institutional buy-in, key representatives of the institutions and units are engaged as members of the research team.
Introduction
The growing prevalence of children with medical complexity (CMC) has important implications for paediatric healthcare, and a profound impact on interactions between parent caregivers and healthcare professionals (HCPs) in the paediatric intensive care unit (PICU).1–4 Advances in medical technology and postoperative care have led to increased survival of CMC who share four defining features: (1) the presence of one or more complex chronic medical conditions that is severe and expected to be lifelong; (2) significant functional limitations typically reliant on medical technology; (3) high healthcare utilisation and (4) high healthcare service needs.5 6 CMC are three times more likely to have an unscheduled PICU admission than previously well children,7 and are at higher risk of prolonged length of stay,1 8 frequent readmission,8–10 adverse events11 and PICU mortality.1 In Canada, they constitute approximately 15% of total PICU admissions, yet are estimated to occupy 25%–50% of PICU beds at any given time.12 The complexity of their care requires a high degree of service coordination and caregiver expertise and has been recognised as a health service priority.13 Often, because of the intensity of their baseline care needs, the care given at home surpasses the level of care possible on general wards, and CMC may require intensive care environments for all of their hospitalisations.
There is growing international recognition of the benefits derived from research that incorporates patient experiences in designing and improving the delivery of family-centred healthcare services.14–16 Although paediatric institutions across North America are committed to supporting patient and family-centred care (PFCC), there has been limited PICU practice change that aligns with these objectives and approaches. One study found PICU nurses’ strict adherence to implicit and explicit unit rules and policies to protect efficient ways of working ran counter to providing PFCC.17 This despite calls over a decade ago by an American College of Critical Care Medicine Task Force to include the family as an integral part of the ICU team based on evidence that PFCC facilitates timely restoration of health, facilitation of patient and family coping, improved patient care, better patient and family healthcare experiences, reduced staff stress related to family interactions and optimisation of the dying process.18 Indeed, neglecting to provide PFCC for CMC may negatively impact child and family health outcomes.
Parents of CMC become technologically sophisticated, expert caregivers who know their child’s medical condition intimately and can detect subtle changes and responses to medical interventions.2 19 20 They challenge the traditional PICU caretaking model where parents assume a relatively passive role, relying heavily on clinician expertise to rescue their child.4 Interventions to promote parental involvement at the bedside have been traditionally based on a PICU model of care that assumes parents lack the knowledge and expertise necessary to provide highly technical care.21–23 HCPs working within that model often fail to take families’ expertise into account despite evidence that parents harbour expectations of a collegial relationship with staff based on continuing negotiation and mutual trust.24 25 When parental and HCP perspectives regarding medical treatment options, care priorities and approaches differ, uncertainty, tension, and conflict regarding caregiving practices and treatment goals may arise.2–4 This collision of expertise can compromise patient care by creating barriers to establishing mutually beneficial partnerships between patients, parents and HCPs that are at the core of PFCC, at a time when children and families are particularly vulnerable.
A new approach to care for CMC is required, in which there is a shift in the existing paradigm towards greater shared expertise models between critical care teams and family caregivers. The educational and practice change interventions designed to implement such a model of care should be rooted in the patient and family experience. The focus of this study is to rigorously determine what the key elements of such a paradigm shift in care, education and practice change ought to be.
Understanding healthcare experiences can yield important insights into care processes. While patient healthcare experiences have become a central indicator of quality healthcare, studies rarely include specific data to guide practice change, resulting in little systematic work in acute care settings to improve those experiences.26 Involving children and parents as knowledge users and equal partners throughout the research process is the central premise of integrated knowledge translation, an approach to enhance research relevancy and maximise the likelihood that findings will be useful to knowledge users, including patients, families and HCPs.27 28 This study will advance our understanding of PFCC experiences, establish priorities for enhancing them, and bring knowledge users together to establish and implement practice change to enhance PICU care.
Objectives of the study
This study aims to develop and assess the implementation of PICU practice changes on PFCC for CMC. Specific objectives include:
To capture and understand how knowledge users (CMC, parents and HCPs) experience PFCC along its four core dimensions (see the Conceptual framework section), in the PICU.
To engage knowledge users as partners in identifying joint priorities for enhancing PFCC.
To engage knowledge users as partners in codesigning and implementing PFCC practice changes.
To evaluate (A) practice changes along the four core dimensions of PFCC and (B) the Experience-based Co-design (EBCD) process.
Conceptual framework
This study is guided by the conceptual framework for PFCC developed by the Institute for Patient-Centred and Family-Centred Care.27 This framework is grounded in mutually beneficial partnerships between HCPs, patients and families that are integrated into the planning, delivery and evaluation of healthcare. Four core dimensions are included: dignity and respect, information sharing, participation in care and decision making, and collaboration. Benefits of PFCC include improved healthcare experiences, better health outcomes, improved care coordination, enhanced patient safety, judicious allocation of resources and improved HCP engagement.29–32 Despite widespread acceptance in principal, acute care settings continue to struggle with integrating PFCC into practice, and little is known about the establishment of effective caregiving partnerships. This is attributed to the continuation of models of care that position parents as visitors at their child’s bedside, rather than integral members of the healthcare team.33 This study will integrate the four core dimensions of PFCC into our data collection approaches, and use them to guide our codesign events and practice change implementation processes as we seek to enhance parent–child–HCP partnerships.
Methods and analysis
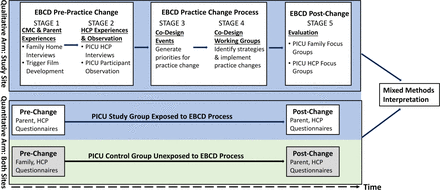
A mixed-methods, concurrent triangulation design will be used (figure 1).34 35 In the qualitative arm, data will be collected at the study site using an EBCD approach.26 36 Through a codesign process, participant knowledge users (ie, CMC, parents and HCPs) will reflect on their PICU care experiences (stages 1 and 2), work together to identify priorities for improving PFCC (stage 3), devise strategies to address these priorities and to implement associated practice changes (stage 4), reflect on and evaluate the practice changes and the EBCD process (stage 5). In the quantitative arm, standardised PFCC-related outcomes will be measured at the study site and at a control site for temporal comparison prior to and following EBCD exposure, and analysed using a difference-in-differences approach.37 Findings from the qualitative arm regarding participants’ perceptions of the EBCD process and resultant practice changes will be interpreted together with findings from the quantitative arm. The complementarity of the qualitative and quantitative data will provide a comprehensive understanding of the impact of practice changes on PFCC.
Mixed-methods, concurrent triangulation design: Experience-based Co-design (EBCD: qualitative arm) and difference-in-differences approach (qantitative arm). CMC, children with medical complexity; HCP, healthcare professional; PICU, paediatric intensive care unit.
Recruitment
Setting
All stages of the qualitative arm will take place at the study site, while the quantitative component will take place at both study and control sites. The two Canadian PICUs participating in this study are located in different provinces, and both are located within university-affiliated paediatric hospitals offering Complex Care Services to CMC. In the qualitative arm, child and parent interviews will take place at the family’s home to optimise comfort (or preferred location), while HCPs will be interviewed in hospital (or preferred location). To minimise risk during the COVID-19 pandemic, interviews will take place via secure video conference. Codesign events, working groups and project team meetings will take place at the study site or via secure video conference according to public health pandemic recommendations at that time. In the quantitative component, parents and HCPs will complete questionnaires at home or in hospital.
Participants and eligibility criteria
CMC <18 years of age who speak English or French will be included with: (1) one or more severe, complex chronic medical conditions expected to be lifelong; (2) significant functional limitations with reliance on medical technology; (3) daily care needs at home similar to hospital care and (4) high healthcare service utilisation, including one or more PICU visits in the previous year.5 Parents of CMC and HCPs who provide direct patient care to CMC in the PICU will be included who speak, read and write English or French.
Quantitative sample
Parents
Prepractice change (time 1), parents of all CMC admitted to the PICU in the 10–12 months preceding the start of practice-change activities will be invited to complete questionnaires. Postpractice change (time 2), we will use continuous sampling and invite all parents of CMC admitted prospectively over the 10–12 month period postpractice change to complete questionnaires. Time periods are based on prior CMC PICU admission numbers at both sites. To examine parents’ perceived changes in PFCC, a sample size of 50 families/group (n=100) at each time point will achieve 80% power at a significance level of 0.05 to detect a medium effect size of 0.638 using a two-sample t-test. We expect at least a medium effect size on the Measure of Processes of Care (MPOC-20), our primary outcome measure, similar to that reported by parents using it to evaluate differences in PFCC services in Canadian healthcare programmes for children with disabilities (see the Measures section).30 Sample size was inflated by 10% as some children will be readmitted and questionnaires will not be independent (based on a 50% readmission rate with a large clustering effect intraclass correlation coefficient=0.15,39 variance inflation factor=1.08).
Healthcare professionals
All PICU and Complex Care staff working with CMC in the PICUs at both sites will be invited to participate during time 1 and time 2.
Qualitative sample
CMC and parents
Maximum variation sampling will be used to select 12–15 CMC and their parents (1 parent/child; n=24–30) from the time 1 quantitative sample who indicated their interest (as per earlier consent) in being contacted for a potential interview. We will aim for variation in child and family characteristics (eg, child age, family structure, ethnicity and socioeconomic status), and medical conditions (eg, age of onset, home care needs, PICU admissions). Analysis and data collection will proceed in tandem and recruitment will continue until data saturation is reached.40 41
Healthcare professionals
Maximum variation sampling will be used to select 12–15 HCPs representing PICU and Complex Care staff of varying levels of seniority and disciplines (with proportional representation from those disciplines, for example, nursing (85%), medicine (13%), respiratory therapy (1%), social work (1%).36 42 Analysis and data collection will proceed in tandem and recruitment will continue until data saturation is reached.40 41
Data collection and analysis
Qualitative arm
Stage 1: Gathering CMC and parent experiences
For parents and children over 9 years of age who agree to be interviewed, written informed parent consent and child assent will be obtained and an interview scheduled at the families’ convenience. A qualitative researcher will conduct video-recorded interviews using a semistructured, narrative approach.40 43 From within the interview narrative, we will seek to identify personal touch points, or key moments during PICU hospitalisation that shaped participants’ PFCC experience.36 44 Participants will be encouraged to describe their experiences of care including interactions with HCPs from their own perspectives, and based on their own care priorities. Experiences will be explored along the four core dimensions of PFCC (online supplemental appendix 1). To ensure analytical rigour, two members of the research team will independently review the video data and analyse professionally transcribed interviews to identify emergent themes through constant comparison analysis.40 They will analyse parent and child data separately when possible. NVivo analytical software (V.12) will be used to support the development of the coding framework, data interpretation and thematic analysis, enhancing the trustworthiness of the data.40 Multiple referents (transcripts, video data) will enhance credibility and confirmability.
Supplemental material
Exemplar quotes from the videorecorded interviews that represent key touch points identified through constant comparison analysis will be included in one composite, 30 min trigger film. Participants will review their own interview and provide written consent and assent for selected clips to be used in the trigger film. They will then be invited along with other family participants to validate the trigger film and identify care priorities prior to its presentation at codesign events (stage 3), where it will be used to trigger discussion and identify joint family-HCP priorities for improving PFCC experiences. The trigger film is a key element of a successful EBCD approach, providing staff with a powerful understanding of patient healthcare experiences.36
Stage 2: Gathering HCP experiences and participant observation
Written informed consent will be obtained from HCPs who agree to participate, and an interview scheduled at their convenience. A qualitative researcher will conduct semistructured, audiorecorded interviews exploring HCPs’ experiences caring for CMC in the PICU, focusing on the core dimensions of PFCC (online supplemental appendix 1). To ensure analytical rigour, two members of the research team will independently review and analyse verbatim, anonymised transcripts to identify emergent themes through constant comparison analysis.40 NVivo analytical software (V.12) will be used to develop the coding framework and support data interpretation and thematic analysis, enhancing the trustworthiness of the data.40 Multiple referents (transcripts, observational field notes) will enhance credibility and confirmability. HCPs will be invited (10 HCPs/group maximum) to review and validate the findings and identify care priorities with an experienced facilitator who is not on staff, prior to presenting data summaries at subsequent codesign events.
Participant observation will provide contextual data to complement participants’ narratives and subsequent codesign work by revealing relational and social processes that may affect PICU care experiences.45 Specific aspects of PICU care will be observed by research staff trained in qualitative methods, including functional (eg, technical care procedures) and relational aspects (eg, settling the child after a difficult procedure). Written informed consent will be obtained from parents and HCPs who agree to participate; child assent will be obtained when possible. To understand PICU activities and care processes and provide insight into significant touch points in healthcare experiences, CMC, parents and HCPs will be observed interacting with one another.36 Observational notes will be read by two members of the research team, and emergent themes identified through constant comparison analysis.40 Thematic summaries will be presented at codesign events to inform discussion of care practices and PFCC priorities.
Stage 3: Codesign events
Parents, children and HCPs who participated in earlier stages of the study and indicated their interest (as per previous consent) will be approached to participate in one codesign event. Should someone decide not to participate, a potential participant will be identified with consideration to maintaining maximum sample variation, and written informed consent or assent obtained. Codesign events will be offered at different time to accomondate participants. CMC, parents and HCPs will view the trigger film together, and an experienced facilitator will then lead discussion of the film and present PFCC priorities identified by families and HCPs in earlier interviews. Smaller, mixed breakout groups will use that information as a springboard to identify joint priorities for enhancing PFCC, focusing on caregiver relationships, information sharing, care coordination and collaboration. Groups will exchange priorities, identify areas of overlap and establish care priorities for CMC. If consensus is lacking across codesign events, a final event with representation from each group will be convened to review differences and determine which priorities to present to the working groups.26 36 44 46
Stage 4: Working groups
Working group participants will be drawn from the codesign events where they will have expressed interest and provided consent to contribute as partners to addressing identified priorities for enhancing PFCC for CMC. Groups will design and implement clinical practice changes. Although it is not possible to identify the precise number of care priorities at the outset of the study, 2–4 working groups (1 group/priority) are anticipated. Each group will consist of 2–3 HCPs, 2–3 parents and 2–3 CMC (9+ years of age). The research team will provide guidance regarding the steps and process and an experienced facilitator will lead one group per care priority to plan a change (plan), coordinate action to implement the change (do), observe and learn from the change (study) and determine required modifications to the change (act) based on the Institute for Healthcare Improvement’s ‘plan-do-study-act’ cycles for creating practice change.47 48 Actions and progress will be documented by a member of the research team assigned to each of the groups to support the process. We anticipate needing approximately three cyles per group to achieve uptake of the desired practice change.
Stage 5: Evaluation
Focused, facilitator-led group discussions will take place to evaluate the extent to which participants perceive that (1) joint priorities for improving the core components of PFCC for CMC were established, and (2) working groups were able to codesign and implement practice changes to address identified care priorities. We will determine what did and did not work during the EBCD process, and explore enablers and barriers to designing and implementing practice change in the PICU. Discussions will be recorded, professionally transcribed and analysed using constant comparison analysis. Key themes identified in the analysis of the data will be reviewed by a subgroup of participants identified in codesign events and working groups to ensure the analysis reflects their experiences.40 Complementary data regarding parents’ and HCPs’ perceptions of engagement in the EBCD process will be collected following stages 3 and 4 using the Public and Patient Engagement Evaluation Tool. This tool was designed to help assess the quality and impact of public and patient engagement activities within health system organisations, and has undergone extensive usability testing.49 Data will be summarised using descriptive statistics, reviewed in conjunction with themes generated from the focus group data, and combined using a parallel data analysis approach50 to consolidate and facilitate data interpretation.
Quantitative arm
Changes in PFCC will be evaluated and a Difference-in-Differences approach used to compare parents’ and HCPs’ perceptions of PFCC at both sites, prepractice and postpractice change (figure 1).37 A letter from the PICU Nurse Manager and Medical Director describing the study will be sent to potential participants via email, along with a personal link to a REDCap secure web platform that will provide study information, a consent form and an online questionnaire. Regular mail will be used to send materials to those who do not have an email address.
Measures
Participants’ demographic and hospital baseline data will be collected from the medical charts (online supplemental appendix 2). Parents will evaluate PFCC using the Refined (MPOC-20, primary outcome measure), a 20-item self-report measure of caregivers’ perceptions of the extent of family-centredness of the care received by children with disabilities in a healthcare organisation.30 The MPOC-20 is composed of five subscales reflecting the core dimensions of PFCC. HCPs will evaluate PFCC using the 27-item MPOC for Service Providers (MPOC-SP).51 Both measures demonstrate good reliability and validity and have been used with parent and HCP caregivers of CMC.
Supplemental material
Analysis
Demographic and hospital baseline data will be summarised at each time point using descriptive statistics. Primary analysis will be based on the MPOC-20, and changes in scores from time 1 to 2 will be compared between sites using the following mixed effect linear regression model: MPOC-20=site + time + site*time [+random effect], accounting for within-patients variation in readmitted patients. For the secondary analyses, changes in MPOC-20 scores from time 1 to 2 will be compared between sites, while further adjusting for patient/parent characteristics (child age, previous PICU admissions, length of stay, risk of mortality, parent education), using a mixed effect linear regression model.52 MPOC-SP data will be similarly analysed, adjusting for HCP characteristics (years of PICU experience, professional role, education).
Mixed methods data interpretation
PFCC practice changes reflected in group differences in time 1 and time 2 MPOC scores (parents and HCPs) will demonstrate the effectiveness of practice changes in enhancing PFCC across its core dimensions, as reflected in the instruments’ subscales. We will use a parallel data analysis approach to consolidate quantitative and qualitative findings.50 Quantitative data will be compared and contrasted with themes generated through constant comparison analysis of parent and HCPs interviews. Since qualitative and quantitative data sets both address similar concepts, we do not expect difficulties integrating them. We will use a mixed-method data matrix to determine where the data sets converge, and where (and why) they diverge to provide a comprehensive interpretation of participant engagement and changes in PFCC experiences for CMC in the PICU.34 35
Patient and public involvement/participation
This study is based on a participatory action research design that integrates knowledge translation throughout all stages of design and conduct of the research. Families were involved in the development of this study and will continue to be engaged in all EBCD events. Knowledge users and researchers will partner throughout the study process.42 53 CMC and their parents will be invited to participate in all EBCD stages in the qualitative arm of this study, and to provide their unique perspectives on PFCC and how it can be fostered in the PICU. The research team will work with the hospitals’ Family Advisory Committees to promote knowledge uptake and dissemination (below).
Ethics and knowledge dissemination
Ethical approval was granted by the McGill University Health Centre Research Ethics Board (Ref. #2019-5021) and the Hospital for Sick Children Research Ethics Board (Ref. #1000063801). Participants will receive verbal and written information regarding the study, and be encouraged to ask questions and discuss their decision to participate. Participants will be told they can withdraw from the study at any time without affecting their hospital care or, for HCPs, their employment. Consent and assent will be revisited and reaffirmed at each stage of the study. Prior to screening the trigger film that will feature video clips of children’s and parents’ interviews, participants will be given an opportunity to view their respective clips and asked to provide written formal consent for their use. No health or safety risks are anticipated; however, participants will be informed that should they experience emotional distress while sharing their experiences and wish to take a break or withdraw from the study, this will be respected. Should they experience ongoing difficulties or concerns after participating and wish to speak with someone, a referral will be made to hospital support staff. Data collection methods respect Federal and Provincial public health regulations regarding COVID-19 restrictions to ensure participants’ safety.
Knowledge dissemination activities will occur throughout the study. Progress will be summarised following each stage in a study newsletter and disseminated to participants, PICU and complex care service staff, clinical and institutional leaders. All participants will be invited to a celebration event at the end of the study to discuss the findings and lessons learnt in the EBCD process. A final report of PICU priorities and recommendations for promoting PFCC for CMC will document key lessons learnt, common solutions and core attributes of the EBCD process that could be transferable across hospital and community care settings. By regularly sharing progress and findings with the hospital Family Advisory Committees at both sites, the dissemination of findings will be promoted locally, provincially and across patient support groups; this will also provide opportunities to explore other means for knowledge uptake. The trigger film will be used for medical and nursing education within hospital and university settings. Presentations, workshops and publications will further ensure dissemination of findings through professional and academic networks.
The growing prevalence of CMC together with the demonstrated benefits of engaging patients and families in service delivery and redesign presents opportunities and challenges for paediatric critical care. CMC present with complex illness trajectories along with expert parent caregivers who know their child’s medical condition intimately, altering the dynamics and established character of HCP–family relationships. Resulting tensions can negatively impact caregiving partnerships in the PICU at a time when they are needed most. This study will advance our understanding of families’ and HCPs’ PFCC experiences, establish joint priorities for enhancing care, and bring CMC, parents, and HCPs together to establish and implement practice changes to enhance PICU care for this vulnerable population.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @janet_rennick, @dryden_palmer
Contributors JR is the principal investigator for the TOPIC CMC study. JR, FB, EC, FC, KD-P, PF, HP, SR, IS-S and SL made substantial contributions to the conceptualisation and design of the study. JR and SL drafted the first version of the manuscript. JR, FB, EC, FC, KD-P, PF, HP, SR, IS-S and SL reviewed the manuscript for intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding This work was supported by the Canadian Institutes of Health Research, grant number 399884.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.