Article Text
Abstract
Introduction Systemic lupus erythematosus (SLE) is a chronic autoimmune disease. SLE is treated with immunosuppressants with suboptimal efficacy and high risk of serious side effects. Patients with SLE have increased risk of mortality, organ damage and debilitating treatment-resistant fatigue. Autonomic nervous system dysfunction (AD) is present in approximately half of the patients and may promote autoimmunity by weakening the vagally mediated anti-inflammatory reflex. Recent studies suggest that transcutaneous vagus nerve stimulation (tVNS) has few side effects and beneficial effects on fatigue, pain, disease activity and organ function. This study investigates whether adjuvant tVNS improves measures of fatigue (primary end point), AD, clinical disease activity, inflammation, pain, organ function and quality of life.
Hence, this study will contribute to the understanding of AD as a potentially important precursor of fatigue, disease activity, progression and complications in SLE, and how tVNS mechanistically may attenuate this. As adjuvant tVNS use may reduce the need for traditional immunosuppressive therapy, this trial may prompt a shift in the treatment of SLE and potentially other autoimmune disorders.
Methods and analysis Eighty-four patients with SLE with fatigue and AD will be randomised 1:1 to active or sham tVNS in this double-blinded parallel-group study. In period 1 (1 week), participants will receive a 4 min tVNS 4 times daily and report on fatigue daily. After a 2-week pause, period 2 (8 weeks) will entail tVNS twice daily and participants will report on fatigue, pain and disease activity weekly. Secondary end points will be assessed before and after each period and after 1 week in period 2.
Ethics and dissemination The study is approved by the Danish Medical Research Ethical Committees (case no: 2120231) and results will be published in international peer-reviewed journals.
Trial registration number NCT05315739.
- rheumatology
- neurophysiology
- immunology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is one of the first studies investigating the effects of transcutaneous vagus nerve stimulation (tVNS) in patients with autoimmune diseases using a randomised, double-blinded, sham-controlled design.
Fatigue is reported as the most frequent, invalidating and burdensome disease manifestation of systemic lupus erythematosus, and thus chosen as a primary outcome.
Compared with previous studies, we will include more and less selected patients, assess effects across the most relevant organ systems, conduct extensive baseline characterisation and explore dose-response qualities of tVNS and thus put tVNS into a clinical context.
tVNS is performed by the patient at home, which limits verification of correct stimulation intensity, duration and anatomical location, but reflects real-life use.
A cross-over design is stronger than a parallel study design, but the latter was chosen to ensure optimal blinding.
Introduction
Systemic lupus erythematosus (SLE) is a systemic chronic autoimmune disease with a heterogenous presentation that may lead to numerous organ manifestations, comorbidities and decreased quality of life.1 The life expectancy of patients with SLE in Denmark is reduced by 25 years compared with the background population,2 and patients with comorbidities including nephritis, neuropsychiatric or cardiovascular diseases have the worst prognosis. For the uncomplicated patient, the 10-year cumulative pure medical costs are roughly €16 000, but increase 10-fold with organ damage.3 Fatigue occurs in >80%4 5 and is reported as the main barrier to maintaining employment in patients with SLE.6 Fatigue and musculoskeletal pain are reported as the subjectively most burdensome symptom for patients with SLE.7 Consequently, SLE has marked impact on morbidity, mortality, healthcare costs and quality of life.
Immunosuppressants, the cornerstone of current care, can have multiple adverse effects, including diabetes, osteoporosis and opportunistic infections,8 9 and may have only limited effect on controlling disease activity,10 fatigue and other constitutional symptoms.7 Thus, alternative treatments that can attenuate autoimmune inflammation and treatment-resistant symptoms with few adverse effects are in demand.
Recent studies suggest that stimulating the autonomic nervous system holds this potential. Autonomic nervous system dysfunction (AD) occurs in a large proportion (54%) of Danish patients with SLE and is characterised by impaired, especially, parasympathetic vagally mediated function.11 AD further relates to a wide range of disease manifestations that are highly prevalent in SLE: fatigue,12 impaired quality of life,11 pain,13 inflammation14 as well as impaired vascular,15 16 cardiac17 18 and renal functions.19 Increasing the parasympathetic vagus nerve activity by transcutaneous vagus nerve stimulation (tVNS) may reverse such consequences of AD. tVNS has decreased fatigue induced in healthy humans20 and in patients with inflammatory rheumatic diseases.21 22 Furthermore, tVNS has improved pain tolerance in healthy humans23 and reduced pain related to cluster headache and migraine.24 25 Additionally, vagus nerve stimulation has been shown to decrease inflammation in animals,26 27 healthy humans28 and patients with systemic autoimmune diseases,21 29–32 which may be vagally mediated via the cholinergic anti-inflammatory reflex.33 Cardiovascular organ dysfunction may be alleviated by tVNS, which can improve microcirculation34 and reduce aortic stiffening35 as well as improve cardiac function in rats36 and human patients.37 All together, this suggests that tVNS may effectively reduce adverse manifestations of SLE.
In contrast to traditional immunosuppressive treatment, tVNS with intended device holds a good safety profile. To the best of our knowledge, no serious adverse events related to this tVNS device have been reported and the most common side effects typically resolve immediately after the stimulation and entail lip or facial drooping (11%), headache (8%), dizziness (3%) and application site discomfort (2.5%).24 38–41
Based on the above, we aim to conduct a comprehensive clinical trial with the hypothesis that adjuvant treatment with tVNS in addition to standard care in patients with SLE improves patient-reported fatigue (primary outcome). Furthermore, we will investigate how tVNS influences other important SLE disease outcomes that reflect the systemic and heterogenic nature of SLE, including AD, disease activity, pain tolerability as well as renal and cardiovascular functions (secondary outcomes).
Methods and analyses
Study design and overview
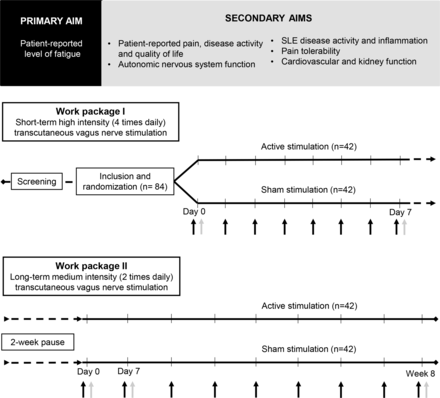
The SLE-VNS study is a 1:1 randomised, parallel-group, sham-controlled investigator-initiated clinical trial. The study is expected to run from May 2022 to ultimo 2024 including data analyses. First participant first visit is expected to take place in May 2022, and last participant last visit in June 2023. The study will be conducted at the Copenhagen Research Center for Autoimmune Connective Tissue Diseases (COPEACT), Rigshospitalet, Copenhagen, Denmark. It is designed with a patient representative (SLE Europe) and in the framework of an ongoing study, investigating tVNS in patients with diabetes with diabetic autonomic neuropathy.42 The study is composed of two work packages (WP; figure 1).
Overview of the systemic lupus erythematosus (SLE)-vagus nerve stimulation (VNS) study.
Work package I
In WP-I, the participants will self-administer either bilateral active or sham tVNS at the cervical part of the vagus nerve 4 times daily for 7 days. The participants will report on fatigue daily in a subject diary, and all secondary outcomes will be assessed at baseline (day 0) and day 7 (figure 1; table 1).
Primary and secondary outcomes, methods and timepoints of assessment
Work package II
After 2 weeks without intervention, all participants will proceed with their allocation into WP-II. tVNS will be self-administered bilaterally 2 times daily for 8 weeks. In weekly online surveys, participants will report on fatigue, musculoskeletal pain and disease activity, as described below (Outcomes and experimental procedures; table 1). Other secondary outcomes will be assessed at baseline (day 0, WP-II), day 7 and week 8 (figure 1; table 1). After all assessments at the final week 8 visit, the participants will be asked whether they believe they received active or sham treatment.
A safety visit is conducted 1 week after cessation of the intervention in WP-II including blood samples and ECG not related to the outcomes of the study.
Study participants
Eighty-four patients with SLE, diagnosed according to the internationally accepted disease classification criteria,43 with signs of fatigue and AD (see inclusion criteria, table 2) will be included.
Inclusion and exclusion criteria
Recruitment and enrolment
Potential participants will be identified at the COPEACT and receive oral and written information about the trial from information screens and leaflets or their regular physician. Screening and inclusion of candidates will be performed by a medical doctor. Eligible participants will have signed the informed consent after meeting all the inclusion criteria and none of the exclusion criteria listed in table 2.
Participants may be discontinued from the study if they are considered non-compliant, withdraw their consent or experience unacceptable adverse events. The discontinued participants will be replaced by new eligible participants in the same treatment arm (active/sham) to ensure sufficient study power.
Baseline characterisation
Participant characteristics will be recorded at WP-I baseline to assess group similarity and allow for stratified responder analyses. The general characteristics will include age, sex, race, height, weight, education, employment status, medication affecting autonomic function and former cardiovascular and other diseases. SLE characteristics will include items from the disease classification criteria,43 Disease Activity Score (DAS),44 damage index45 and immunosuppressive medication such as antimalarials, corticosteroids and synthetic and biological disease-modifying antirheumatic drugs (DMARDs). Finally, biochemical and immunological evaluations will be performed as part of the SLE characterisation, including autoantibodies against double-stranded DNA, SSA, SSB (Sjogren's Syndrome A and B), U1RNP (U1 ribonucleoprotein particle), Smith antigen, cardiolipin, beta-2-glycoprotein, lupus anticoagulant and direct agglutinin test unless documented within the previous year.
Intervention
The active tVNS device
tVNS will be carried out with the handheld, battery-powered gammaCore Sapphire device (electroCore, New Jersey, USA) that sends electrical signals through the skin and soft tissue of the neck to activate the vagus nerve. The device is a class IIa medical device and is CE marked (CE 571753) for: (a) acute and/or prophylactic treatment of certain primary headaches (migraine, cluster headache and hemicrania continua) and medication overuse headache; (b) treatment or prevention of symptoms of reactive airway disease; (c) adjunctive therapy to reduce the symptoms of certain anxiety and depression conditions; (d) adjunctive therapy in the prevention of partial onset and generalised seizures associated with epilepsy and (e) adjunctive therapy to reduce the symptoms of gastric motility disorders and irritable bowel syndrome.
Stimulation with the device is provided through two steel contact electrodes covered with conductive gel (Sigma gel, Parker Laboratories, New Jersey, USA). When activated, the device produces a proprietary low‐voltage electrical signal comprising a 5 kHz sine wave burst lasting for 1 ms. Bursts are repeated once every 40 ms (25 Hz), generating a 24 V peak voltage and 60 mA peak output current. On activation, the electrical current is transmitted for 120 s. The intensity of the stimulation is adjusted by the user in the range of 1–40 arbitrary units via the digital user interface.
Sham device
Sham tVNS will be administered by a sham device identical to the active device in appearance and application. The sham device can, however, not produce electrical stimulation on activation but provide a light ‘vibrational sound’ to mimic the active treatment.
Instruction
The participants will be thoroughly instructed in the use of the device by research personnel, who is not otherwise involved in the study to minimise any risk of unblinding. Accordingly, the participants will be instructed to retain from sharing information about the sensation of the treatment to the study personnel. A Danish user guide and a subject diary will be handed out along with the device. The participants will be instructed to perform daily self-administered stimulations during the two WPs. During the initial instruction session, the participants will be instructed to position the device at the cervical course of the vagus nerve, anteriorly to the sternocleidomastoid muscles and laterally to the carotid arteries. The correct placement will be marked with a permanent marker on the skin and the participants will be encouraged to refresh the markings throughout the trial and take a picture of the location. The participants will receive their first treatment during the instruction session to ensure correct use.
Interventional stimulation
During WP-I, participants will perform four stimulation doses daily (every 6 hours), and during WP-II, only two stimulation doses daily (every 12 hours). Each stimulation dose consists of bilateral tVNS: 120 s to each vagus nerve. The participants will be instructed to use the highest tolerable stimulation intensity and note the intensity and time of each stimulation in the subject diary.
The tVNS will be applied as an add-on treatment to the participant’s standard of care immunosuppressing medication. If clinically indicated, this medication can be changed during the trial, and these changes will be recorded.
Outcomes and experimental procedures
The outcomes and methods of assessment are summarised in table 1 and described in detail below.
Primary outcome
The Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) scale is a validated 13-item questionnaire that assesses patient-perceived fatigue and its impact on daily activities and function over the past 7 days.46 It has been used in numerous clinical SLE trials, has superior internal consistency and higher sensitivity compared with other fatigue measures47 48 and is, thus, included as the primary outcome measure of fatigue.
Other patient-reported outcomes
The Composite Autonomic Symptoms Score questionnaire will be applied to provide a quantitative measure of the participants’ self-reported AD symptoms.49 The participants’ self-reported SLE disease activity will be evaluated with the Systemic Lupus Activity Questionnaire50 and the Patient Global Assessment.51 Furthermore, the participants will assess the average musculoskeletal pain on an 11-point visual analogue scale.52 Quality of life will be evaluated with the validated 12-item short form (SF-12) questionnaire, derived from the original SF-36,53 and a physical and mental component score of patient-reported health-related quality of life will be calculated.
Autonomic nervous system function
The visit-based tests of autonomic nervous system function will be undertaken in the morning in a quiet room according to recommended protocol,54 where smoking, food and caffeine intake are restricted prior to testing.
Resting autonomic function will be assessed in four ways: (1) a 5 min resting heart rate variability (HRV) will be measured with the handheld ECG Vagus device (Medicus Engineering, Aarhus, Denmark)55; (2) 5 min resting cardiac vagal tone will be measured with the non-invasive ECG eMotion Faros device (Mega Electronics, Kuopio, Finland)56; (3) after the 5 min rest, blood pressure and heart rate will be measured with standard equipment and (4) stimulated sweat secretion will be measured as the electrochemical reaction mediated by chloride ions after stimulation of sweat glands in hands and feet with the non-invasive SUDOSCAN device (Impeto Medical, San Diego, California, USA).57
Cardiovascular autonomic reflexes will be assessed in two ways: (1) by three consecutive heart rate-based cardiovascular reflex tests with the Vagus device, in which the ratio of the maximal and minimal beat-to-beat intervals in relation to standing, deep breathing and the Valsalva manoeuvre are compared with age-dependent cut-off levels to assess the degree of AD: no, early (one abnormal) and manifest (more than one abnormal test) dysfunction58 and (2) by assessment of orthostatic blood pressure changes with the participant standing for 5 min after supine rest and blood pressure measurements each minute.
Continuous autonomic function will be assessed with a small patch sensor Holter device (ePatch, BioTelemetry Technology, Hørsholm, Denmark) that records a 3-lead ECG for seven consecutive days.59 Participants will press a button on the device just prior to the tVNS, leaving a location mark in the dataset that allows for HRV analyses in relation to the tVNS. Time and frequency domain HRV parameters will be calculated based on the ePatch and Vagus measurements.60
SLE disease activity
Disease activity will be evaluated by clinical and laboratory examination according to three different activity scores (SLE Disease Activity Index-2000 (SLEDAI-2K), SLEDAI Responder Index-50% (SRI-50) and SLE-DAS). The SLEDAI-2K44 is most commonly used for activity assessment, whereas SRI-50 accounts for clinically significant improvements between visits,61 and SLE-DAS is suggested to have improved sensitivity to change and specificity compared with the SLEDAI-2K.62 Furthermore, the physician’s judgement of overall disease activity will be scored in the Physician Global Assessment (PGA)63 by answering “How do you rate your patient’s current disease activity?” with mild=1 to 3=most active disease imaginable. The physician-assessed number of painful and swollen joints according to DAS64 will be evaluated. Finally, based on the medication history, any changes to the patient’s regular SLE medication will be noted throughout and until 3 months after the study. Anti-inflammatory medication will be grouped into the following groups: antimalarials, glucocorticoids, synthetic and biologic DMARDs. Changes will be analysed based on introduction, termination and dosage of drugs during the course of the study.
Pain tolerability
The tolerance to sensory pain stimuli will be assessed with bone and muscle pressure with a handheld pressure algometer (type 2, Somedic Production, Sweden) and a circulating ice-chilled water (2℃) bath. At first, the algometer will apply pressure (30 kPA/s) to the tibia and quadriceps muscle. Thereafter, the hand will be immersed into the water for 120 s or until the pain becomes intolerable. Pain intensity will be rated regularly by the visual analogue scale during the immersion. Immediately after the immersion, the quadriceps muscle pressure will be reapplied, which allows for quantification of the conditioned pain modulation capacity.65
Organ function
A transthoracic echocardiographic ultrasound examination (LOGIQ S8, GE Electronic) will be performed in order to assess cardiac geometry, ventricular mass, diastolic and systolic function.66 Arterial stiffness will be assessed with pulse wave velocity measured by ECG traced pulse-wave Doppler ultrasound at the carotid and external iliac artery.67 Furthermore, microvascular morphology will be assessed by in vivo nailfold video capillaroscopy with the Dino-Lite digital microscope (Vodskov, Denmark), revealing both the architecture of capillary rows and fine details of each vessel.68 To characterise renal function, urine and blood samples will be analysed for estimated glomerular filtration rate and urinary protein-creatinine ratio.
Biochemical and immunological function
Routine: SLE biochemical status based on plasma and serum routine analyses will be performed to assess changes relevant to disease activity and other disease properties.
Experimental: to asses immunological function, the following will be measured: (a) plasma cytokines reflecting inflammatory activation and inhibition, (b) interferon-regulated gene expression (nCounter platform, NanoString Technologies, Seattle, Washington, USA), (c) immune cell population distribution in whole blood (fluorescence-activated cell sorting) and (d) functional immune cell stimulation (TruCulture). To characterise the effects on metabolic control, plasma lipid and glucose profiles will be performed.
Randomisation and blinding
Included participants will be provided with a unique randomisation ID number. The collaborative site at Aalborg University Hospital will be responsible for the block-randomisation (eight participants) with www.randomization.com. The randomisation list will be kept at Aalborg Hospital, and only sealed envelopes containing the treatment allocation for each participant will be kept at a secure location at the COPEACT for individual unblinding in case of medical emergencies. Hence, all personnel involved in the study and participants will be blinded to the randomisation. Following the last participant’s last visit, a blinded dataset divided into treatment ‘A’ and ‘B’ will be prepared for all outcomes to allow for blinded data analyses.
Adverse events
The participants will be instructed to report on adverse events at every visit and to contact the research personnel during WP-I and WP-II if adverse events arise. All adverse events will be recorded in the case report form (CRF). A physician investigator will assess all adverse events for causality with tVNS. Study personnel must immediately report any serious adverse event or serious adverse device effect to the primary investigator. All device effects will be reported to the manufacturer yearly and any serious adverse events within 7 days. Additionally, all adverse events and effects will be reported to the Danish medical research ethical authority after the study end. Based on occurrence of serious adverse events, the primary investigator will be able to terminate the study. The participants will be covered by the regular patient insurance during their participation in the trial.
Data collection and data management
Data will be collected by experienced research personnel trained in good clinical practice (GCP) and entered to electronic CRFs using RedCAP Electronic Data Capture Tool pertaining to the given approval by the Danish Data Protection Agency (P-2022-114). Data from physical questionnaires and participant diaries will be entered manually to the electronic CRF by two different researchers to limit errors. Digital source data from, for example, image-based or autonomic outcomes will be saved on a secure drive with the participant identification number and analysed blinded after trial end. Blood and urine samples will be labelled and stored in a secure research biobank for analysation after trial end and stored for a maximum of 10 years. All other experimental data will be entered directly into the CRFs. Digitalised data will be backed up and stored for 5 years under the responsibility of the principal investigator, whereas physical CRFs with source material will be kept at a secure location for 5 years.
Data analysis
The primary outcome will be analysed by intention-to-treat approach, meaning that all randomised participants will be included in their initially assigned study arm regardless of adherence to study protocol. Changes in the primary outcome measure will be compared between the two groups by Student’s t-test. Secondary end points will be analysed by per-protocol approach by general linear modelling of repeated measures and application of relevant post hoc analyses or Fisher’s exact test as appropriate. The potential effect of differences in baseline values and possible unblinding will be investigated by appropriate adjustments in general linear models or stratified analyses.
For all analyses, p≤0.05 will be considered statistically significant. The applied statistical program will be SPSS statistics (V.25, IBM).
Sample size calculation
This study is powered to detect a minimal clinically important difference of 5.9 points on the FACIT-F scale69 between the active and sham tVNS-treated groups after 1 (WP-I) or 8 (WP-II) weeks of stimulation. Based on a mean±SD baseline score of 20±8.0,70 29 participants per group are required with the use of the intended significance level to provide a statistical power of 80%. With allowance of a 30% dropout rate, we aim to include 42 participants in each arm.
Monitoring
Internal monitoring will be conducted weekly to ensure that the protocol, national regulations and GCP standards are followed. The monitor will review source documents and medical records to confirm CRF-recorded data and will monitor all signed informed consent documents and adverse events logs. Quality assurance audits by relevant regulatory authorities may be performed.
Patient and public involvement
The study outcomes were discussed and chosen in collaboration with a SLE patient representative. Instead of choosing an objective measure as primary outcome, we chose patient-reported fatigue as the primary objective of the study, as it is highly prevalent and burdensome in SLE and an objective measure may not correlate with patient evaluation and satisfaction with the treatment.
After study completion, the participants will be informed on their study allocation (active/sham), and study results will be disseminated to relevant patient associations. No public involvement was included in the design phase of the study.
Ethics and dissemination
The study protocol has been approved by the Danish Medical Research Ethical Committees (case no: 2120231). The study will be performed in accordance with this published protocol and the registration at ClinicalTrials.gov, the principles of GCP (DS/EN ISO 14155:2020), the guidelines of the revised Helsinki Declaration and applicable local regulatory requirements and laws.
All publication rights belong to the principal investigator. Positive as well as negative and inconclusive trial results will be published in international peer-reviewed journals. A primary author will be subscribed according to the Vancouver system.
Discussion
This study was designed to provide novel substantial evidence on the effect of tVNS on fatigue in SLE. The design further allows for a detailed and comprehensive description of effects on other disease manifestations relevant to patients with SLE.
In other patient populations, tVNS has ameliorated manifestations frequently observed in SLE. Unfortunately, only few of the studies have been systematically controlled, and until recently, the implications of tVNS treatment of patients with SLE remained undescribed. Interestingly, a recent randomised, double-blinded, sham-controlled pilot study of 18 patients with SLE showed attenuating effects on pain, fatigue and number of swollen joints following 4 days of 5 min auricular tVNS.70 However, the study only included few and highly selected participants with high levels of musculoskeletal pain and disease activity and followed the participants for 12 days. Furthermore, the study did not find effects on other markers of inflammation and disease activity. We speculate that power and follow-up length may influence these results. Hence, we aim to complete a comprehensive study that could account for this.
The current study holds the overall strength that it aims to put tVNS into a clinical context. This will be done by (a) including participants that represent the majority of patients with SLE, as fatigue and AD are common in SLE; (b) conducting extensive baseline characterisation that will enable identification of markers related to possible tVNS responders and (c) providing extended follow-up and assessment of dose-response qualities of tVNS, which should give insights to dynamic of tVNS effects. All together, these factors could help facilitate clinical implementation if tVNS is found effective. Supplementary to the primary outcome, this study will also investigate the effects of tVNS across the most relevant organ systems implicated in SLE. This will give insights to the prospect of using tVNS as an alternative to the current standard treatment with immunosuppressants. Furthermore, this may enable a better understanding of the diverse clinical picture presented by patients with SLE and the pathophysiological mechanisms of fatigue, AD and inflammatory activity, which hitherto is poorly described.
The study does hold some limitations. We will not be able to verify whether each active stimulation is performed correctly, as the treatment will be self-administered at home. Therefore, participants will undergo a thorough introduction and perform the first stimulation under supervision, including emphasis on the correct device position by marking it on their skin, and every stimulation will be logged in diaries. Also, there is a risk of some participants guessing if they receive sham treatment based on missing signs of muscle and skin nerve activation. To quantify the latter, subjects will be asked about this after completion of the study. The chosen sham method was, however, judged the best possible comparator. To optimise the blinding and overall study quality, the treatment will be tested in a parallel-group design, the tVNS participant instruction will be performed by a person not otherwise engaged in the study in a similar manner regardless of allocated treatment arm, and participants will be instructed to refrain from sharing information about the sensation of the treatment to study personnel. As for randomisation method, the time for the effects of tVNS to fade should be considered but has not previously been investigated. In the SLE pilot study, the effects of tVNS on fatigue and pain remained 7 days after the intervention.70 Therefore, randomising the order of the WPs could be advantageous. However, as the study is conducted in the framework of another study to allow for comparison with effects of tVNS in patients with diabetes, we chose the current study design.
With this study, we aim to provide novel clinical evidence about the effects of tVNS on fatigue and other important clinical and paraclinical manifestations of SLE. This study may contribute to the introduction of a safe and effective treatment of SLE as an alternative or supplement to the current standard of care immunosuppression. Such treatments would constitute a paradigmatic shift in the care of patients with SLE and other chronic inflammatory diseases.
Data statement
Within the limitations of the national regulations on data sharing and after the publication of trial results, the data generated can be provided in anonymised form on reasonable request from researchers who provide a methodological sound proposal.
Ethics statements
Patient consent for publication
Acknowledgments
We express our gratitude towards Jullie Rudnicki, Anne-Marie Wangenberg and Kasper Yde Jensen for aiding project logistics and methodologic sparing during the design phase of the trial.
References
Footnotes
Contributors AHZ: conceptualisation, methodology, validation, formal analysis, investigation, writing—original draft, writing—review and editing, visualisation, supervision, project administration, funding acquisition. ILD: investigation, writing—original draft, writing—review and editing, visualisation. KAM: methodology, writing—review and editing, project administration. AF: methodology, writing—review and editing, supervision, project administration, funding acquisition. MP-J: conceptualisation, methodology, validation, resources, writing—review and editing, supervision, funding acquisition. CB: conceptualisation, methodology, validation, resources, writing—review and editing, supervision, project administration, funding acquisition. SJ: conceptualisation, methodology, formal analysis, resources, writing—review and editing, visualisation, supervision, project administration, funding acquisition.
Funding This work is supported by the Danish Rheumatism Association (Gigtforeningen) (R198-A7026, R209-A7483); the Per Henriksen Foundation; Helsefonden (22-B-0460); Grosserer LF Foghts Foundation (22.103); Aase and Ejnar Danielsens Foundation (22-10-0120) and Rigshospitalets Foundation (R245-A10816-B709). Applications for further covering of running expenses and analyses with various other foundations will be submitted ongoingly.
Disclaimer The funding sources have not had and will not have any involvement in study design, collection, analyses and interpretation of data, writing of the report or decision to submit the subsequent article(s) for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.