Article Text
Abstract
Introduction Persistent infection with high-risk human papillomavirus (hrHPV) is the main cause of cervical cancer. Thus, the effective treatment against HPV represents an opportunity to reduce the incidence of cervical cancer. Although various treatments are effective in treating HPV infection, they still provide limited benefit in reducing the rate of cervical cancer due to the lack of implementation of a standardised protocol in many low/middle-income areas. This proposed cohort study aims to describe the status quo of treatment, attributions of the treatment decision-making process and potential factors influencing treatment decisions.
Methods and analysis This is a mixed-method, 5-year prospective longitudinal study in Lueyang County, China, one of the areas with the highest cervical cancer incidence rates and lowest mean income in China. We will enrol women with hrHPV infection (at least one HPV type in the 13 high‐risk subtypes) diagnosed via a county-wide HPV infection and cervical cancer screening programme. The study procedures describe the treatment patterns and explore the potential influencing factors in treatment decision-making through questionnaires, laboratory examinations and in-depth interviews. All participants will be evaluated at baseline and at 6, 12, 24, 36, 48 and 60 months. The primary outcome is the treatment pattern, the type and duration of which will be described later. The secondary outcomes include guideline compliance and changes in the HPV infection status. The HPV impact profile, intimate relationship satisfaction, and costs within different management groups are also described and compared.
Ethics and dissemination This study was reviewed, and all of the relevant approvals were obtained from the Ethics Committee of the Maternity Service Centre of Lueyang Maternal and Child Health Care Hospital (2021-001). The findings from this study will be disseminated through peer-reviewed publications, conference presentations and academic workshops.
Trial registration number ChiCTR2100053757.
- Public health
- EPIDEMIOLOGY
- Infection control
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
To our knowledge, this is the first study to consider real-world data regarding the management patterns after diagnosing high-risk human papillomavirus infection among women in China.
We will use a mixed method to explore different potential factors, revealing their internal influence mechanism on treatment decision-making options.
Implementing this protocol will identify the gap between research and practice and determine why some patients did not receive the recommended therapies.
It remains a challenge to sustain a high participation rate in a prospective cohort study; working outside the hometown and insufficient knowledge and awareness about the disease in Lueyang County would lead to the loss of participant follow-up.
This is a community-based observational study of prospectively collected data from Lueyang County, China, so the study design increases the risk for the potential limitations of the presence of selection bias and a decreased generalisability of the findings to other populations.
Introduction
Cervical cancer ranks fourth among malignant tumours in women worldwide,1 presenting a serious threat to women. Cervical cancer is a major public health concern.2 Globally, there was an estimated incidence rate of 15.3 per 100 000 and a mortality rate of 7 per 100 000 for cervical cancer in 2018.3 In China, the incidence rate is 10.88 per 100 000, and the mortality rate is 3.17 per 100 000.4 Cervical cancer arises from four processes: human papillomavirus (HPV) infection, persistent HPV infection, multistage squamous intraepithelial lesions and invasion through the basement membrane.5 The WHO is developing a global strategy, known as the 90–70–90 triple intervention strategy, for eliminating cervical cancer as a public health problem by 2030.6
Currently, great progress has been made in the prevention of cervical cancer (eg, HPV vaccination and cervical screening initiatives) and the development of several therapies for cervical cancer. For women who are already HPV infected, the WHO guidelines recommend safe and effective treatment options, but these do not reach the women who need these services the most.7 8 Several factors contribute to this failure in health service delivery, access and utilisation, including a lack of a link between screening and treatment (compliance with treatment and follow-up), variability in service quality and insufficient continuing education for service providers.9 10 Thus, the implementation of the standardised management of high-risk HPV (hrHPV) infection or HPV-caused pre-cancerous lesions to reduce the prevalence of cervical cancer urgently needs to be implemented.
Treatments for HPV infection and HPV-induced pre-cancer lesions include conservative observation, ablative treatment (cryotherapy and thermal ablation) and excisional treatment (excision with a cold knife cone (CKC) and electrosurgical excision (Large Loop Excision of the Transformation Zone (LLETZ) or Loop Electrosurgical Excision Procedure (LEEP)).7 11 Several studies have shown that patients with HPV infection or benign lesions can undergo tissue destruction by thermal (hot or cold), electrical or chemical means, the success rates for which vary widely, and the variability of the inclusion criteria for each study hampers establishing a standardised management protocol.8 12 The quality of evidence for all outcomes is low to very low, and the level of heterogeneity is high in all pooled analyses. For instance, although all currently used ablative techniques are effective in reducing cancer risk, there is a lack of data on the long-term effectiveness of therapies, reasons for treatment failure and cost-effectiveness of therapies.
For these reasons, there remains a lack of sufficient real-world evidence to support clinicians and patients in providing optimal treatment decision-making recommendations. Various factors can shape treatment decisions, including decision-maker characteristics, decision-specific criteria and contextual factors.13 14 The first are the decision-maker characteristics, including those for both the providers and patients, such as capabilities (ie, knowledge of HPV), emotions (ie, worry, anxiety or stigma from HPV infection) and degree of expertise (ie, benefits and limitations of each treatment).15 16 Second, decision-specific criteria involve classic clinical criteria, such as age, HPV type, duration of infection, sex or expected treatment complications (ie, adhesion or bleeding).17 Contextual factors include the patient’s socioeconomic status, healthcare system, treatment costs and perceived support (ie, intimate relationships).18 Currently, it is not yet clear which patient factors affect treatment decision-making after testing positive for HPV.
The All-China Women’s Federation and the Ministry of Health launched the ‘Two Cancers’ (cervical cancer and breast cancer) screening programme in July 2009, which implemented a free cervical cancer screening programme for rural women aged 35–64 years. This programme involved publicity, health education and examinations.19 In recent years, with the popularisation of cervical cancer screening, the incidence of cervical cancer in some areas of China has been effectively controlled. Lueyang County is a low-income area in China with high morbidity and mortality rates for cervical cancer. The incidence of cervical cancer has shown a decreasing trend in recent years; however, the morbidity and mortality of the disease remain high. The high prevalence rate of HPV infection (18.5%) in this region was higher than the national overall prevalence rate of HPV infection (15.54%).20 We still do not know why morbidity or mortality is higher, and how to provide services to patients in this county. Furthermore, the standard of care for HPV and cervical lesions provided in China and how it deviates from the WHO recommendations remain unclear.
Thus, we propose establishing a prospective cohort for women infected with hrHPV in Lueyang, Shaanxi, aiming to (1) profile the treatment patterns and adherence to guideline-concordant management for hrHPV infection in the real-world setting, (2) identify the characteristics associated with treatment decision-making for hrHPV infection, (3) explore the reasons for treatment choices, including initial regimen, switch and treatment termination, and (4) evaluate the long-term effects of different treatment approaches. The results will provide real-world evidence to support optimal decision-making in the treatment of hrHPV infection and, ultimately, strive to achieve the goal of ‘90% of women with pre-cancer treated’, which was proposed by the WHO in its Cervical Cancer Elimination Initiative.
Methods and analysis
Study design and setting
This mixed-method, prospective cohort study with a 5-year follow-up will take place in Lueyang County, one of the economically underdeveloped regions in Shaanxi Province, China. Lueyang County has a high prevalence of HPV infection. All women between 18 and 65 years old in the county are invited to participate in a local government-supported cervical screening programme. The cervical screening services are managed by gynaecologists at the Maternity Service Centre of Lueyang Maternal and Child Health Care Hospital. The study was approved by the Maternity Service Centre of Lueyang Maternal and Child Health Care Hospital, and it was started on 4 November 2021 and is planned to be completed by 30 November 2026.
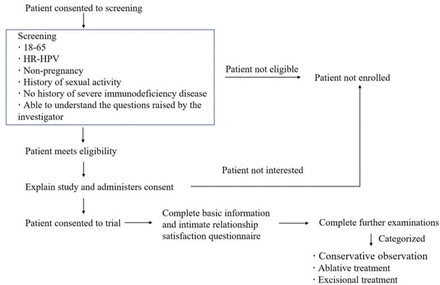
During the screening phase, women aged 18–65 years are recruited through a cervical screening service by gynaecologists and nurses. Basic information, hrHPV testing and inclusion evaluations will be performed. The women diagnosed with hrHPV infection during the screening phase will be invited to participate in the study. Written informed consent will be obtained from the women regarding their participation in this prospective cohort study (including the procedures, risks and options for dropping out of the study). Medical staff with adequate training will clearly explain the study protocol and objectives to the participants. After providing consent, each patient must sign an informed consent form. Subsequently, a participant identification (PID) number will be assigned to facilitate the study, and the other examinations will be performed. In the categorised phase, according to the outcomes of examinations and treatment decision-making, all patients will be divided into conservative observation, ablative treatment and excisional treatment groups. Figure 1 shows the recruitment process that will be used.
Workflow for the recruitment procedure. HR-HPV, high-risk human papillomavirus.
Eligibility criteria
The inclusion criteria are as follows: (1) women aged 18–65 years, who had lived for 5 years in Lueyang County, (2) a diagnosis of hrHPV infection (including 13 subtypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68; participants with at least one HPV type in the high-risk group) in the screening phase, (3) non-pregnancy, (4) history of sexual activity, (5) no history of severe immunodeficiency disease and (6) able to understand the questions asked by the investigator.
The exclusion criteria are as follows: (1) refusal to participate in this study, (2) a diagnosis of cervical cancer, (3) other malignant tumours or serious illness, and (4) a diagnosis of mental illness or impaired consciousness.
Definition of outcomes
This study will assess the treatment patterns, compliance with guidelines and HPV status (for detailed information, see online supplemental table S1).21–24 Patients in the conservative observation group will undergo routine follow-up.25 The patients will be observed without any additional treatment. Ablative treatments include cryotherapy and thermal ablation.6 Abnormal tissue is destroyed by heating with thermal coagulation or freezing with cryotherapy. Excisional treatment involves the surgical removal of abnormal tissue with LLETZ or CKC.6 Switching was defined as a change to a different treatment before completing the assigned course of treatment. The switch could be to any treatment (observation, ablative or excisional treatment). The time-to-switch was defined as the period from the date of the first treatment regimen to the date of switch to another treatment regimen during the study period.
Supplemental material
Sampling strategy
All women meeting the inclusion criteria will be recruited for the study and followed up for 5 years. Management patterns will be described using proportion and 95% two-sided exact CIs. With an estimation that among the total 40 000 women aged 18–65 years in Lueyang County, 18.5% will be diagnosed with hrHPV infection, a total of 7500 women will be eligible for the study. Considering a 20% (up to 40%) attrition rate,26 we will end up with 6000 (at least 3000) women in the cohort. Since the proportion of patients’ treatment choice is unknown, varying proportions produce different actual widths as follows (see online supplemental table S2), the CI width was determined by PASS (V.15) with the following parameter settings: CI formula, exact (Clopper-Pearson); confidence level (1−α), 0.95; proportion, 0.1–0.9 by 0.1. A priori, we determined that feasibility would be confirmed with (1) a recruitment rate of >60%26 and (2) attrition rate of <40%.
Data collection
The data collection protocol will be performed according to the list outlined in table 1. All data will be obtained by physicians at the hospital. The participants underwent a comprehensive physical examination and completed a questionnaire through face-to-face interviews. All biochemical test results (HPV testing, Thin-prep liquid-based cytology test, colposcopy and pathological examination) and sexually transmitted diseases (syphilis and HIV) will be obtained from the hospital records. Follow-up visits will be conducted at 6, 12, 24, 36, 48 and 60 months.
Data collection schedule
Measures
Baseline
The baseline data include the individual information, characteristics and laboratory data. Individual data will be collected through a health interview survey and medical records. Demographic variables, such as age, education level, occupation, annual income, ethnicity and religion, will be used in the analysis, as data concerning smoking status, alcohol consumption, sexual history (total number of sexual partners, frequency of sexual life and forms of contraception), HPV vaccination history (type of vaccine and vaccinated person-time) and hygiene habits (ie, frequency and manner of bathing). Other basic information, such as menstrual history (ie, time to menarche/menopause), marital history, HPV knowledge (HPV infection, regular examination and HPV vaccination), physicians’ knowledge regarding treatment (benefits and limitations of all treatments), partner information and gynaecological examinations (ie, lesions of the vulva, vagina, vaginal secretions, cervix, cervical polyps, uterus, uterine accessories, vaginal cleanliness and sexually transmitted disease type), will also be assessed. All eligible patients will undergo gynaecological examinations.
Outcomes
Laboratory data, clinical characteristics and use of therapies will be assessed in the hospital, and all laboratory examinations will be performed during the non-menstrual period. All outcomes will be collected by gynaecological outpatient doctors, and the diagnoses will be made by a specialised gynaecological pathologist. Treatment patterns, as the primary outcome, will be collected by receiving the available data on the history of therapies from all eligible patients. Secondary outcomes include the clinical characteristics (HPV status, cytology, colposcopy, biopsy results and adverse events) and other assessments (HPV impact profile, intimate relationship satisfaction and costs; see online supplemental table S2). The specific examination procedures were shown in figure 2.27
The specific examination procedures.27 ahrHPV, high-risk human papilloma virus; bTCT, Thin-prep liquid-based cytology test; c≥ASC-US, atypical squamous cells of undetermined significance or above; dNILM, negative for intraepithelial lesions or malignancy; eLSIL, low‐grade squamous intraepithelial lesion; fHSIL, high‐grade squamous intraepithelial lesion.
Other assessments
The HPV impact profile, a self-reported scale, is designed to represent the full spectrum of potential HPV-related impacts.28 29 An intimate relationship satisfaction questionnaire, a self-made satisfaction questionnaire, is employed to estimate the satisfaction of patients with HPV infection. Direct and indirect costs will be estimated using medical information30 related to cervical screening, diagnosis, and treatment of HPV and cervical lesions. The details are provided in the online supplemental table S3.
A semistructured interview guide (see online supplemental table S4) has been developed for women with hrHPV infection and service providers. The initial questions on the guide will help explore participants’ perceptions and attitudes towards HPV infection. Additional questions on the guide will assess the impact of these perceptions and attitudes on treatment choices, treatment switching and non-adherence to guidelines. All semistructured interviews will be conducted face-to-face or via phone. Interviews will be scheduled based on the participant’s convenient day and time. Interviews are anticipated to begin on 1 August 2022.
Quality control
The investigators received training on the standard operating procedure before patient recruitment was performed. A standardised operation process manual and operation video were created and distributed to all of the research assistants. There will be a question-and-answer session to solve operation problems after approximately 10 patients are enrolled. In addition, the research assistants will provide feedback on a daily basis for patients who had submitted problems within the working group.
Data management and analysis
All participants will be allocated a PID code at enrolment, which will be used in the samples and documents over the 5-year study period. All data will be collected and managed using an electronic data management platform protected by the firewall of Chongqing Medical University. The data on the platform will be accessible only by authorised researchers using private accounts and passwords. Any change will be automatically recorded in the platform log and saved as a separate file for data monitoring purposes. For the data export process, the de-identification of patient health information will be conducted following the HIPAA (the Health Insurance Portability and Accountability Act) rule. An independent safety monitoring board will be established when the study begins and will monitor safety throughout the study period. The data will be verified by double-checking for erroneous or missing values, and data analysis will be performed using SAS V.9.1.3 (SAS Institute).
The demographic and clinical characteristics of the participants will be summarised using the mean and SD for continuous variables. For categorical variables, the proportion (%) will be reported. Significant associations in the contingency tables (cross-tabulations) will be assessed using standard Pearson’s χ2 test. Analysis of variance will be used to compare differences in the continuous variables among the three groups. The patient demographics, clinical characteristics, treatment therapies (to identify the most common treatments and duration) and guideline adherence will be descriptively reported. In addition, we may apply growth mixture modelling to observe whether subsets of individuals follow distinct trajectories over time. Analyses were performed for the entire cohort and stratified by treatment patterns, occurrence of switching and HPV types (16/18 or other 11 subtypes).
Management patterns will be described as the proportion and 95% exact CI (95% CI). For this method, p will be the population proportion, and r represents the number of successes from a sample of size n. Let pˆ=r/ n. Exact test (Clopper-Pearson) using a mathematical relationship31 between the F distribution and the cumulative binomial distribution, and the lower and upper confidence limits of a 100(1−α) % exact CI for the true proportion p are given by:

Multivariate logistic regression models will be used to estimate the potential factors influencing the treatment choices, including the personal information (sexual history, HPV vaccination history (type of vaccine and vaccinated person-time)), decision-maker characteristics (patient and physician), specific criteria (13 hrHPV subtypes, duration of infection and adverse events) and contextual factors (patient’s socioeconomic status, treatment costs and perceived support (ie, intimate relationships)). Pearson’s χ2 statistics will be used to compare the treatment differences in clearance, recurrence and persistence rates among the groups.
Qualitative data will be transcribed verbatim and analysed using thematic analysis.32At this stage, a bottom-up inductive approach will be used to identify patterned meanings in the dataset within an essentialist framework to report the experiences, meanings and realities of the participants. NVivo software (V.11, QSR International, Doncaster, Australia) will be used to import, organise and explore the data for analysis. An iterative process will be employed to label data and identify emerging themes. To ensure inter-rater reliability, two independent investigators will perform the coding, category creation and thematic analyses. The team will liaise several times to review themes and subthemes, resolve discrepancies, and decide on the final definitions of themes and subthemes.
At each follow-up, the proportion of patients who received guideline-concordant care will be assessed. The proportion of patients having experienced an event at specific time points (6, 12, 24, 36, 48 and 60 months) will be derived from the switching rates. Kaplan-Meier curve will be used to estimate the median time-to- switch with relevant treatment events as a treatment switch, 95% CIs for median times to event will be computed. Multivariate analysis of the switching factors (decision-maker characteristics, decision-specific criteria and contextual factors) will be tested using Cox regression analysis.
Pearson’s χ2 statistics will be used to compare the treatment differences in clearance, recurrence and persistence rates among the groups. The Kaplan-Meier method will be used to construct the cumulative clearance rate of HPV from the date of the first HPV diagnosis to the date of HPV clearance in each treatment group. A proportional hazards model will be fitted to evaluate the effects of treatment options and other predictors on the overall persistence and recurrence of HPV, and possible interaction terms of the main effects will be tested by comparing a reduced model with the full model. Adverse events will be compared among the different groups. Data will be reported as medians and IQRs or as numbers and percentages. All comparisons will be evaluated using Wilcoxon signed-rank test with continuity correction.
Missing data will be identified and reported as percentages. We will also include several other key parameters (discount rate, annual number of screened women, screening positivity rate, biopsy rate and programmatic costs) in the one-way sensitivity analysis.
Patient and public involvement
The patients and the public were not involved in the development of the research questions or the design and analysis of the study. The extent of patient involvement in the study included answering the survey questionnaires at baseline and each follow-up. The results will be disseminated to the applicants in the form of a published article, which will be made available upon request.
Ethics and dissemination
Ethical approval for the study was obtained from the Biomedical Research Ethics Committee of the Maternity Service Centre of Lueyang Maternal and Child Health Care Hospital. All participants fulfilling the inclusion criteria will commence as full ethics approval is received from the Biomedical Research Ethics Committee. No other independent ethics reviews were conducted. The results of this study will be disseminated in peer-reviewed journals and conferences. We will provide clinicians with feedback following the peer-review process to further strategise optimal treatment decision-making.
Discussion
Women are currently concerned about cervical cancer, owing to its increasing incidence and high mortality in low/middle-income areas. Despite the availability of safe and effective methods for treating pre-malignant conditions, real-world evidence of treatment effects in low/middle-income areas remains limited. To our knowledge, there are no real-world data regarding patients’ treatment patterns after being diagnosed with hrHPV infection among women in these regions, and the characteristics that affect their decision-making process are rarely reported. In this study, we focus on the characteristics of clinical outcomes in different treatment groups and the relationship between decision-making and the individual’s characteristics in terms of individual behaviours, psychological trajectories and economic burden. Hence, this study is able to evaluate how these factors influence the patient’s decision-making process. It will also allow us to study the research-to-practice of discordance in greater detail with the ultimate goal of uncovering the underlying causes of discrepancies between these formal recommendations and current practices.
Decision-making has acquired crucial importance in disease management over the last 20 years since the introduction of the patient-centred principle. A variety of factors can shape treatment decisions, which include decision-maker characteristics, decision-specific criteria and contextual factors.13 Previous studies have stated that there are a variety of factors that influence treatment decisions in oncological diseases and other diseases, such as capabilities, emotions, age, expected treatment risk, the healthcare system and treatment costs.18 Little is known about the predictive factors for decision-making in women undergoing hrHPV testing. This study intends to include different influencing factors to reveal their internal influence mechanism on decision-making options. The qualitative findings of this study will help us explore the perceptions and attitudes toward HPV infection and its impact on the individuals’ treatment choices. Moreover, we will gain an in-depth understanding of gynaecological care needs, which will aid us in developing context-specific programmes for patients infected with hrHPV in low socioeconomic areas in future. We expect that the results can inform clinical practice with the provision of predictive algorithms that provide physicians with a clear profile of the patient at present and their probable trajectory in the long term.
This study has several strengths. First, it provides a comprehensive understanding of the therapeutic status quo in hrHPV infection and the possible underlying mechanisms involved, including the treatment patterns and disease outcomes under different treatment decisions. Second, this prospective cohort study has a long follow-up period. Therefore, this study may assist in determining causal associations between the predicted determinants of treatment decision-making and can be used to obtain trajectories of how patients’ conditions influence decision-making over time.33 Describing and analysing time-invariant and time-variant factors that predict improvement or deterioration in these trajectories will help identify potentially modifiable risk factors for future interventions, as well as patients at risk of poor outcomes. Third, this study identifies the gap between research and practice and why some patients did not receive recommended therapies. It provides an effective mechanism to ensure high compliance to treatment and also provides an opportunity to reduce cervical cancer incidence and mortality in low/middle-income areas.
High participation rates at follow-up are critical for the validation of cohort studies. A challenge we anticipate is the loss of follow-up, which is generally a common failure in longitudinal studies. The 6-month, 12-month, 24-month, 36-month, 48-month and 60-month follow-up appointments may remain difficult to sustain for the following reasons: (1) some adults in rural areas leave their hometowns to work in cities, which means some data could not be collected promptly; (2) insufficient attention paid by patients, which might be related to the insufficient knowledge and awareness about the disease reported in China.34 Maintaining follow-up visits is a challenge we anticipate. A research assistant will communicate regularly with the recruited participants through WeChat, the most popular messaging and calling app, allowing one-to-one communication. The research assistant will confirm the appointment dates and send out reminders the week prior. We will offer payment to cover transport expenses and meals on the day of the visits to help increase the adherence of the participants to the protocol schedule. Discordant results and previous treatments will be recorded, and their treatment regimen will depend solely on their behaviours. After this descriptive study, we will provide health education to raise women’s treatment adherence and clinicians’ guideline adherence. During the study, participants diagnosed with cervical cancer will be referred to anti-cancer treatments but will continue follow-up assessments, noting the change in their disease status.
Overall, we can explore treatment patterns and their association with individual characteristics and unravel the changing trajectory of potential factors that affect decision-making. Most importantly, new real-world insights will be provided regarding the role of tailored disease management in the prevention and treatment of hrHPV infections. These results imply that standardised management could help to reduce the prevalence of cervical cancer in the future. These data may help inform future clinical trial designs, highlight the need for better adherence to treatment guidelines and inform clinical decision-making.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
SY and LB are joint first authors.
Contributors QS, SY, RZ and WX contributed to the study design. LB, DH, RZ, SY, YN and RX performed the study. SY and LB drafted the initial manuscript. QS and SY revised the draft. All authors have read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.