Article Text
Abstract
Objectives To explore the outcomes of Helicobacter pylori infection treatments for naïve patients in the real-world settings.
Design A retrospective observational study.
Setting Single tertiary level academic hospital in China.
Participants We identified patients initially receiving quadruple therapy for H. pylori infection from 2017 to 2020 in whom eradication was confirmed (n=23 470).
Primary outcome Efficacy of different initial H. pylori infection treatments.
Secondary outcome Results of urea breath test (UBT) after H. pylori eradication.
Results Among 23 470 patients who received initial H. pylori treatment, 21 285 (90.7%) were treated with amoxicillin-based regimens. The median age of the patients decreased from 2017 to 2020 (45.0 vs 39.0, p<0.0001). The main treatments were therapies containing amoxicillin and furazolidone, which had an eradication rate of 87.6% (14 707/16 784); those containing amoxicillin and clarithromycin had an eradication rate of 85.5% (3577/4182). The date of treatment, age, antibiotic regimen and duration of treatment showed correlations with the failure of H. pylori eradication in a multivariable logistic regression analysis. Finally, positive UBT results after eradication clustered around the cut-off value, in both the 13C-UBT and 14C-UBT.
Conclusions The major H. pylori infection treatments for naïve patients were those containing amoxicillin and furazolidone, which offered the highest eradication rate. The date of treatment, age, antibiotic regimen and duration of treatment were risk factors for the failure of H. pylori eradication. Additionally, positive UBT results after eradication clustered around the cut-off value.
- gastroduodenal disease
- gastrointestinal infections
- infectious diseases
- diagnostic microbiology
Data availability statement
Data are available upon reasonable request. Data are available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This observational retrospective study is based on a large clinical dataset, to avoid bias and ensure comprehensiveness.
All data were extracted from an electronic medical record system, to ensure authenticity and relatively high completeness.
The findings lack generalisability due to limitations of the data source; this was a single-centre study.
Some data were inevitably missing, as the treatment protocol could not be strictly enforced.
Introduction
Helicobacter pylori is a gram-negative bacterium with prevalence varying from 24.4% to 70.1% worldwide; it accounts for over a third of global infection-attributable cancer cases.1 2 H. pylori infection results in gastric diseases like chronic active gastritis and peptic ulcer disease, as well as extragastric diseases including heart diseases.3 As H. pylori infection remains a major public health issue, the antibiotic resistance of H. pylori has increased alarmingly.4 Fortunately, the reinfection rate remains relatively low.5 Therefore, the effectiveness of initial H. pylori therapy is crucial because the rate of eradication failure increases with two or more rounds of treatment.6
Although the prevalence of H. pylori infection in mainland China exhibited a slow decline of around 0.9% per year in the past decades, it is still widespread.1 7 Successful treatment is defined as a ≥90% eradication rate.8 The preferred empirical therapy for H. pylori infection in China,9 that is, bismuth-containing quadruple therapy, achieved an eradication rate of 87.3% in East Asia in a recent meta-analysis.10 Meanwhile, resistance of H. pylori has been increasing in recent years, and resistance to clarithromycin is considered a major cause of the failure of clarithromycin-based therapy.4 11 12 However, the eradication rate for susceptibility-guided therapy with clarithromycin is promising, at >95%.13 The outcome of clarithromycin-containing therapy in real-world practice remains uncertain.
The urea breath test (UBT) is the preferred non-invasive method to detect H. pylori infection for initial diagnosis and assessment after treatment.14 The principle of UBT is based on the highly active urease enzymes produced by H. pylori, which catalyse the reaction of a labelled urea molecule into labelled carbon dioxide that can be detected in breath samples.15 13C-UBT and 14C-UBT have shown similar sensitivity and specificity.16 13C-UBT can be used in children and pregnant women, while 14C-UBT is contraindicated for these populations because of its radioactivity.17 It is widely acknowledged that results close to cut-off values are not reliable.9 Setting a low cut-off value improves sensitivity while specificity remains high.18 It is suggested that the cut-off value should also be set according to the timing of UBT, that is, before or after the eradication treatment. In most studies, UBT results in the ‘grey zone’ were not common,19 which differs from clinical practice. Thus, the cut-off value for UBT after H. pylori eradication should still be set in light of new evidence.
In this study, we aimed to provide an overview of the management of H. pylori infection based on a large clinical dataset. The aim was to elucidate the ongoing changes in the diagnosis, treatment and outcomes of H. pylori infection, to offer fresh insight into management strategies.
Materials and methods
Study design and population
Patients diagnosed with H. pylori infection who received initial proton pump inhibitor (PPI)-bismuth-containing quadruple treatment between 1 January 2017 and 31 December 2020 were identified by searching the electronic medical records of the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China). Patients with a positive biopsy for H. pylori or UBT were diagnosed with H. pylori infection. Patients were excluded if they had previously undergone H. pylori eradication treatment, experienced a change in regimen during therapy, did not have their H. pylori infection status confirmed after eradication or had incomplete clinical data. We retrospectively collected data from the patients’ medical records, including age, sex and treatment-related variables (date of treatment, regimen, treatment duration and H. pylori eradication outcome). Data extraction was performed in September 2021. Patients’ data were deidentified and two researchers checked the data independently. Online supplemental file 1 shows the detailed study protocol.
Supplemental material
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans for this research.
Exposure
Gastroenterologists inquired about treatment-naïve patients’ history of antibiotic exposure before prescribing treatment, and determined the treatment based on their clinical experience. The prescription was recorded in the electronic medical record system. We focused on bismuth-containing quadruple therapies (PPI+bismuth+two antibiotics), which were recommended as the main empirical therapy for H. pylori eradication in China.9 Different first-line treatments were classified into seven categories, and PPIs into six categories, according to the fifth Chinese National Consensus Report on the management of H. pylori infection (online supplemental file 2).
Follow-up and outcomes
Follow-up was performed through outpatient clinical visits. Patients were asked to visit the outpatient clinics over a period of at least 4 weeks after completion of H. pylori therapy. Eradication of H. pylori infection was confirmed by 13C-UBT or 14C-UBT at least 4 weeks after therapy. 14C-UBT was contraindicated in children and pregnant women because of its radioactivity. Patients were informed about the similar sensitivity and specificity of 13C-UBT or 14C-UBT and the different costs and contraindications due to radioactivity. The patients chose the treatments themselves. The cut-off value for 13C-UBT was 4.0‰ (delta over baseline, DOB), and that of 14C-UBT was 100 (disintegrations per minute). Patients were not permitted to take any PPIs 2 weeks prior to the UBT, or any antibiotics 4 weeks before the UBT.
Univariate and multivariable logistic analyses
A binary logistic regression analysis was performed to examine the relationship between the failure of H. pylori eradication and various factors. Patients who did not receive regimens with amoxicillin plus furazolidone, amoxicillin plus clarithromycin or furazolidone plus clarithromycin were not included in the analyses. Patients who received 12-day treatment were also excluded from the analyses. Covariates were included in the multivariable model when their p values were <0.1 in univariate analysis, when adding the covariate to the model changed the OR by >10%, or on the basis of previous findings. After verifying the stability of the results among different models, we derived the final model using the forward stepwise method (likelihood ratio; criterion for model inclusion and removal=0.05 and 0.10, respectively).
Statistical analyses
Non-normally distributed continuous variables are presented as median (IQR) and categorical variables as absolute frequencies (proportions). The primary outcome was the H. pylori infection eradication rate. Continuous variables were compared using the non-parametric Kruskal-Wallis test. Categorical variables were compared using the χ2 test. Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS (V.26.0) and GraphPad PRISM software (V.9.0; GraphPad Software, Inc., San Diego, California, USA).
Results
From January 2017 to December 2020, 25 796 naïve patients diagnosed with H. pylori infection received PPI-bismuth-containing quadruple therapy and took a UBT for at least 4 weeks after the treatment. Among those patients, 23 470 (91%) were included in the analysis (figure 1). Most of the patients (90.7%, 21 285/23 470) were treated with amoxicillin-based regimens.
Study flow chart. UBT, urea breath test.
Baseline characteristics
The patients’ baseline demographic and clinical characteristics are presented in table 1.
Baseline characteristics
Time-trend analysis
Online supplemental figure 1A depicts the age distribution among people who received H. pylori eradication treatment from 2017 to 2020. Bimodal distributions existed for all groups, with an increase in the number of young patients occurring over time. The median age was 45.0 (33.0–54.0) in 2017, 40.0 (31.0–54.0) in 2018, 39.0 (30.0–53.0) in 2019 and 39.0 (30.0–54.0) in 2020. Online supplemental figure 1B shows relative proportions of different UBTs used; an increase in the use of 14C-UBT over time can be seen, from 42.2% in 2017 to 59.6% in 2020 (p<0.05).
Efficacy results
The overall H. pylori infection eradication rate rose considerably from 83.8% in 2017 to 86.8% in 2020 (online supplemental table 1). Figure 2A shows that amoxicillin-based therapies achieved a higher cure rate than amoxicillin-free therapies every year during the time frame (85.5% vs 70.1%, p<0.001 in 2017; 88.3% vs 70.8%, p<0.001 in 2018; 86.7% vs 77.4%, p<0.001 in 2019 and 87.7% vs 75.8%, p<0.001 in 2020). Figure 2B depicts the eradication rate of three dominant regimens by date of treatment. The eradication rate of therapies containing amoxicillin and furazolidone was higher than that of therapies containing amoxicillin and clarithromycin in 2017 (87.0% vs 78.9%, p<0.001). However, there was no significant difference between these two therapies in later years (88.5% vs 86.9%, p=0.178 in 2018; 86.7% vs 86.9%, p=0.814 in 2019 and 88.1% vs 86.1%, p=0.112 in 2020). During the 4-year period, therapies containing furazolidone and clarithromycin had the lowest cure rate (63.1% in 2017, 66.3% in 2018, 75.4% in 2019 and 75.1% in 2020). The high eradication rates of the other therapies might be inconsistent with real-world practice due to the small sample size (online supplemental table 2).
Efficacy results. (A) The eradication rate of amoxicillin-based regimens and amoxicillin-free regimens. (B) The eradication rate of three dominant therapies by date of treatment. (C) The eradication rate by age. (D) The eradication rate of two dominant therapies by age. A, amoxicillin; C, clarithromycin; F, furazolidone.
The eradication rates were 89.5%, 87.2%, 85.6%, 83.3% and 80.4% in patients aged ≤30, 31–40, 41–50, 51–60 and >60 years, respectively (p<0.001, figure 2C), indicating a higher eradication rate among younger patients. The same age trend was also observed for therapies containing amoxicillin and furazolidone, and for those containing amoxicillin and clarithromycin (figure 2D). In patients aged ≤30 and 51–60 years, therapies containing amoxicillin and furazolidone had better outcomes than therapies containing amoxicillin and clarithromycin (90.8% vs 87.8%, p=0.002 and 85.2% vs 81.8%, p=0.020). Patients aged >60 years old had the lowest cure rate with both kinds of therapies (82.8% vs 78.9%, p=0.052).
We also analysed how the treatment duration and UBT result before treatment impacted the H. pylori eradication (online supplemental figure 2). Generally, there is little significant difference between 10-day and 14-day therapies with amoxicillin and furazolidone. For therapies including amoxicillin and clarithromycin, 14-day treatment provided a better result than 10-day treatment in 2019 (88.8% vs 82.8%, p=0.002), but no significant difference was observed in 2020 (84.6% vs 88.4%, p=0.233). The UBT value before treatment was approximately the same regardless of whether the eradication succeeded (p>0.05, online supplemental figure 3).
Multivariable logistic regression analysis of the failure of H. pylori eradication
We used a logistic regression model to identify factors predicting the failure of H. pylori eradication (table 2). We derived the final model using the forward stepwise method (likelihood ratio; criterion for model inclusion and removal =0.05 and 0.10, respectively; Hosmer and Lemeshow test statistic, p=0.652). The multivariable analysis showed that age, date of treatment, antibiotic regimen and treatment duration were associated with the poor outcomes, while sex, season and PPI use were not.
Univariate and multivariable analyses of risk factors for H. pylori eradication failure
Specificity of UBT after H. pylori eradication
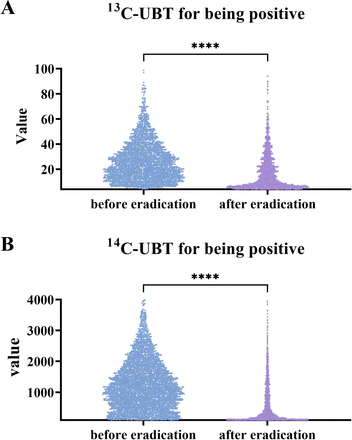
Figure 3 shows the data of positive 13C-UBT and 14C-UBT for naïve patients before and after eradication treatment. The median UBT value for patients positive before eradication treatment was much higher than that after treatment (23.40 (14.30–34.90) vs 12.30 (6.50–24.60) for 13C-UBT, p<0.0001; 1118.0 (636.0–1702.0) vs 303.0 (146.0–930.0) for 14C-UBT, p<0.0001). The results for positive UBT patients after eradication clustered around the cut-off value, but this is not seen for those with negative results (online supplemental figure 4).
Results of UBT for being positive before and after H. pylori eradication. (A) The scatter plot of positive 13C-UBT results before and after H. pylori eradication. (B) The scatter plot of positive 14C-UBT results before and after H. pylori eradication. The cut-off value of 13C-UBT was 4.0‰ (delta over baseline), and that of 14C-UBT was 100 (disintegrations per minute). UBT, urea breath test. **** represents P < 0.0001.
Recurrence after confirmation of H. pylori eradication with stricter criteria
Successful H. pylori eradication with stricter criteria was determined based on the UBT performed at least 8 weeks after the end of the initial H. pylori eradication treatment.5 Recurrence was determined based on a UBT result >2.5 times higher than the cut-off value after successful eradication. Among 10 056 patients for whom H. pylori eradication was successful, 1617 retook the UBT (23 had qualitative but not quantitative data). The 13C-UBT result for 16 of 843 individuals was >10‰, and the 13C-UBT results for 16 of 751 individuals was >250 (online supplemental table 3). The overall recurrence rate was 2.2%. For patients who received the amoxicillin-furazolidone and amoxicillin-clarithromycin regimens, the recurrence rates were 1.8% and 2.1%, respectively; there was no significant difference between these rates.
Discussion
In this large-scale retrospective study, we present the outcomes and follow-up data of initial H. pylori treatments performed over a 4-year period in patients seen at a single centre in East China.
The recommendation that H. pylori-positive individuals receive early eradication treatment, to benefit both themselves and society, led to a shift in practice.20 The indication for H. pylori eradication were also expanded in China, where eradication is recommended for confirmed H. pylori infection cases.9 We observed that the age of patients decreased over time. There were two main age clusters, that is, young and middle-aged. The risk profiles of these two groups differed, such that they were treated via two different strategies.
From 2017 to 2020, H. pylori treatments included 21 285 amoxicillin-based regimens and 2185 amoxicillin-free regimens. The eradication rate of amoxicillin-free treatments was much lower than that of amoxicillin-based treatments. Amoxicillin is considered as a major component of H. pylori treatment in case of low resistance.4 Doctors should carefully investigate documented patient allergies to penicillin. Previous reports indicate that most patients who claim to be allergic to penicillin ultimately have a negative skin test.21–23 Moreover, H. pylori might correlate with the occurrence and persistence of chronic spontaneous urticaria,24 which could result in false positive skin tests. In addition, some patients mistook adverse reactions, such as nausea, for allergy. Detailed information should be recorded to help us identify the truly allergic patients. Furthermore, only one fatal case of anaphylaxis in the UK between 1972 and 2007 was potentially associated with oral amoxicillin.25 Delabelling penicillin allergy is currently of great concern, and direct challenge might be a safe and effective approach.26 Based on the existing evidence, physicians should have more confidence in the safety of oral amoxicillin.
Amoxicillin treatment schemes involving furazolidone or clarithromycin are the most widely used, and are both generally prescribed as 14-day regimens. We could discern an effect of an updated guideline not recommending levofloxacin for initial treatment (ie, few prescriptions thereof).9 H. pylori remains highly sensitive to amoxicillin, furazolidone and tetracycline in China, especially East China.12 Antibiotic regimens with amoxicillin and furazolidone dominated in the past few years, as tetracycline was not available in our hospital pharmacy. However, furazolidone is not used in some countries despite its low resistance. The Federal Drug Agency warns that furazolidone may reduce fertility or injure unborn children.27 Nevertheless, the International Agency for Research on Cancer classified furazolidone into group 3, that is, not carcinogenic in humans.28 The Shire company stopped marketing furazolidone, and eventually withdrew it because of poor sales.29 According to a meta-analysis, a 14-day furazolidone-containing regimen with a low daily dose of 200 mg was well-tolerated and should be used as first-line treatment.30 No serious adverse events were reported among the cases in our study. Furazolidone-containing therapies with a high eradication rate should be re-evaluated in other countries.
Clarithromycin resistance has increased in the Asia-Pacific region in the past few decades, presumably due to the increasing consumption of macrolides.31–33 Clarithromycin-containing regimens are not recommended in areas where clarithromycin resistance exceeds 20%.32 However, the effectiveness of regimens with amoxicillin and clarithromycin was similar to that of regimens with amoxicillin and furazolidone from 2018 to 2020. According to updated guidelines, the gastroenterologists in our hospital were instructed to inquire about the patients’ history of antibiotic exposure before prescription, which might explain the contradictory results. This suggests that we should further investigate regimens involving clarithromycin for H. pylori eradication, and focus on patient populations for whom they may be effective. In a population with high resistance to clarithromycin, metronidazole and levofloxacin, susceptibility-guided therapies and a highly effective empiric regimen both achieved eradication levels >95%.13 The latter treatment should be preferred, considering its simplicity. Given the controversy over the empiric regimen of choice in our region, further prospective studies are warranted for this scenario.
Factors associated with eradication failure in this study included the date of treatment and duration, patient age and antibiotic regimen. Patients who received therapies during the period 2018–2020 were less likely to experience eradication failure than those in 2017, when the new expert consensus report was published. There might be a relationship between eradication outcomes and clinicians’ knowledge of clinical practice guidelines. Moreover, failure of H. pylori eradication was more likely in older patients. However, the ‘test and treat’ strategy is not recommended for young children; it is considered unnecessary until middle-school age Japan, and in those aged ≤14 years in China.9 14 34 However, screening among high-school students and undergraduates might be an important measure to improve the eradication rate, reduce the risk of gastric cancer and prevent transmission, although a lower eradication rate has also been reported in younger patients, especially those with gastric ulcers.35 Symptoms and endoscopic and pathological findings suggest varying pathological mechanisms of H. pylori infection. Thus, these factors should be assessed in future studies to determine the relationship between age and eradication outcomes. Consistent with the recommendation of the Maastricht V/Florence Consensus Report that the treatment duration of bismuth quadruple therapy be extended to 14 days,17 our work showed a slight positive correlation between the treatment duration and outcome. However, the difference between the two major therapies was not significant; this should be further investigated.
In agreement with a prospective study, we observed no association between the UBT value before treatment and H. pylori eradication status.36 Also, patient outcomes were not significantly different according to the PPI used. However, a meta-analysis reported higher cure rates with new-generation PPIs (esomeprazole and rabeprazole) than first-generation PPIs (omeprazole, lansoprazole and pantoprazole), especially in CYP2C19 extensive metabolisers.37 Other factors such as adherence to treatment, cigarette smoking and genetic factors, also played a role.38 39 These factors should be further explored in future investigations.
UBT is recommended after H. pylori eradication, with the monoclonal feacal antigen test serving as an alternative.9 However, the monoclonal feacal antigen test was not available in our hospital until November 2021. After radioactive drugs containing 1 μCi of carbon-14 urea were approved,40 the use of 14C-UBT increased over time, although less than predicted considering its economic benefits. Unexpectedly, after eradication treatment, UBT results close to the cut-off value were not uncommon in our cohort. Long-term follow-up of 13C-UBT results after H. pylori eradication suggested that a lower cut-off value may improve diagnostic accuracy, based on the changes seen in the gastric density of microorganisms.18 Negative UBT results were not clustered around the cut-off, in contrast to the positive ones. This might lead to misclassification of the eradication failure and underestimation of the eradication rate. However, the stool antigen test is even less accurate for patients after H. pylori eradication, and thus has lower positive predictive value than the UBT.41 42 Further studies are needed to address this important but overlooked issue.
This study had several limitations. First, retrospective studies do not permit definite conclusions to be drawn, and some bias is inevitable. Furthermore, the follow-up schedule was not as strict as would have been the case in a prospective study. Second, patient information was incomplete. Factors such as prior antibiotic exposure, resistance to antibiotics, treatment compliance/adherence, smoking history, the H. pylori infection status of family members, socioeconomic status and hygiene status were not analysed. Third, although there are other first-line treatment regimens for H. pylori infection,10 we only focused on therapies containing PPI and bismuth, especially amoxicillin-based therapies including furazolidone or clarithromycin. Vonoprazan, as a potent new acid inhibitor, has not yet been approved for H. pylori infection in China.43 Vonoprazan-based therapies achieved eradication rates >90%, indicating promise for H. pylori infection treatment.
With improved understanding and greater public attention, the treatment options for H. pylori infection, especially for young people, might increase. Amoxicillin-free regimens accounted for 9.3% of the treatments in our cohort. Doctors should be aware of the importance of amoxicillin and correct concept of penicillin allergy. Regimens involving amoxicillin and furazolidone were the most widely used among our cohort, presumably due to the generally acknowledged increasing resistance of H. pylori to clarithromycin and levofloxacin. However, the observed effectiveness of quadruple therapy containing amoxicillin-clarithromycin contradicts this. Notably, 13C-UBT and 14C-UBT results after H. pylori eradication clustered around the cut-off value, which suggests the need to review current mainstream diagnostic methods. Further studies to confirm the effectiveness of different regimens, and the specificity of UBT for H. pylori diagnosis, are needed.
Data availability statement
Data are available upon reasonable request. Data are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank all the physicians and participants who contributed to this study. The authors thank Textcheck for editing the original manuscript (reference number: 22080607).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Correction notice This article has been corrected since it first published. The affiliation of the second author, Yu Xiang has been updated.
Contributors Conception and design: QD, JY and YWang. Acquisition of data: YWang, YX, OL, YWu and YL. Analysis or interpretation of the data: YWang, YX and JY. Drafting of the manuscript: YWang and JY. Revision of the manuscript: JY, QD and YL. Study guarantor and supervision: QD and JY.
Funding This work was supported by grants from the National Natural Science Foundation of China (No. 81773065); Natural Science Foundation of Zhejiang Province (No. LY21H160023).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.