Article Text
Abstract
Objectives This study aimed to determine the impact of meibomian gland dysfunction (MGD) on quality of life and psychosomatic conditions.
Design This was a clinic-based cross-sectional study.
Setting This study was conducted at the eye clinic of the University of Cape Coast, Ghana.
Participants 215 clinical subjects visiting the clinic for a comprehensive eye examination.
Primary and secondary outcome measures Symptomatic MGD, asymptomatic MGD, quality of life scores, depression, anxiety and stress.
Results 215 clinical subjects consented to participate in the study, but 212 were included in the analysis. The mean age was 21.9 (± 3.8) years, 54 had MGD and 158 did not have MGD served as controls. There was no statistically significant difference in the mean quality of life scores between subjects with MGD and subjects without MGD (t=1.57, p=0.12). The quality of life scores (DEQS) (p=0.022) were significantly higher in the symptomatic MGD group compared with the asymptomatic MGD group. There was no significant difference in quality of life scores (DEQS) (p=0.251) in the asymptomatic MGD group compared with healthy controls. Using Pillai’s trace in the MANOVA, there was a significant effect of MGD on depression, anxiety and stress (V=0.05, F(3,208)=3.76, p=0.012). Furthermore, Pillai’s trace in the MANOVA showed a significant difference between asymptomatic and symptomatic MGDs for depression, anxiety and stress scores stress (V=0.24, F(3, 51)=5.24, p=0.003).
Conclusion The study revealed no difference in the quality of life scores between MGD and non-MGD groups. However, the symptomatic MGD group had worse quality of life and psychosomatic symptoms than the asymptomatic MGD group and non-MGD group.
- BACTERIOLOGY
- Strabismus
- Medical retina
- Glaucoma
- Cataract and refractive surgery
- Corneal and external diseases
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- BACTERIOLOGY
- Strabismus
- Medical retina
- Glaucoma
- Cataract and refractive surgery
- Corneal and external diseases
Strengths and limitations of this study
The study looked specifically at the impact of meibomian gland dysfunction (MGD) on a clinical sample’s quality of life and mental health.
The study included a reasonably large sample of a population with MGD.
The study was conducted in a relatively younger population; hence, the results may not reflect the aged population.
The study was conducted in a clinical sample hence may not reflect the situation in the general population.
The study was cross-sectional and hence could not provide the long-term impact of MGD on quality of life.
Introduction
Meibomian gland dysfunction (MGD) is the leading cause of dry eye disease worldwide.1 It is characterised by terminal duct obstruction and qualitative/quantitative changes in glandular secretion.1 It may be associated with eye irritation and ocular surface discomfort.1 2 MGD is a prevalent condition causing increased evaporation of the tears and thereby instigating tear hyperosmolarity, which is a significant factor for developing ocular surface discomfort.1–4 In addition, the lid changes that occur during the pathogenesis of MGD, such as telangiectasia, lid margin thickening, lid notching, pouting of gland orifices and eyelash malposition, can lead to symptoms of ocular irritation often occurring in these patients.2 3
Apart from being the leading cause of dry eye disease, MGD is a frequent cause of posterior blepharitis, implying that MGD impacts the ocular surface and lid margin significantly.2 5–7 Furthermore, studies have shown that MGD is increasingly present in patients reporting visual discomfort.8 For instance, Fenga et al9 showed that 52 (74.3%) of 70 visual display terminal users reported significant visual discomfort and had MGD. Also, studies have shown an association between MGD and lid wiper epitheliopathy, a common cause of eye irritation without apparent ocular surface damage.10 11
Though closely related to dry eye disease and posterior blepharitis, MGD is a distinct clinical entity whose impact on quality of life need to be assessed. Recent studies have shown that MGD frequently occur in a youthful population but may be underdiagnosed because most MGD is non-obvious obstructive MGD and typically not apparent to the examining clinician except on careful examination of the meibomian glands.5 12 Studies on quality of life and ocular surface disease have focused on dry eye disease and allergic conjunctivitis.13 14 Studies on the impact of MGD on quality of life are scarce in the literature and non-existent for the younger population.
The concept of quality of life is quite broad and comprises many domains, including but not limited to the psychological, physical, social, family, vision-related and environmental domains.15 Assessment of these primary domains can unearth the impact of MGD on a patient’s quality of life. Consequently, treatments for MGD are ultimately influenced by the perceived improvement in quality of life than the mere improvement in the objective clinical measures of MGD. Studies have shown that dry eye and rhinoconjunctivitis adversely impact quality of life, and both conditions have been associated with psychosomatic disorders such as depression.13 16 17 Studies have not yet looked at the potential impact of MGD on psychosomatic symptoms and quality of life.
Several treatments are emerging for MGD,18 and it is imperative to know if MGD has a significant impact on quality of life to ascertain whether treatment of the condition will be commensurate with an improvement in the patient’s quality of life. To achieve this objective, it is essential to establish if MGD impacts adversely on the quality of life and psychosomatic conditions. Unfortunately, no studies have explored the impact of MGD on the younger population’s quality of life and mental health. Therefore, this study aims to ascertain the association and impact of MGD on quality of life and psychosomatic symptoms.
Methods
This study was a clinic-based cross-sectional study. Consecutive patients attending the University of Cape Coast Eye clinic for an eye examination were invited into the study. Subjects were included in the study if they were aged 17–40 years; were not undergoing any surgical or cosmetic ophthalmic procedures; not taking medications known to affect meibomian glands such as isotretinoin, hormone replacement therapy or bioidentical hormone therapy; no known psychiatric disorder such as schizophrenia; not using any systemic medications known to improve meibomian gland function such as azithromycin and doxycycline; not having Sjogren syndrome and connective tissue disease (such as rheumatoid arthritis); not undergone haematopoietic stem cell transplantation; and not using any prebiotics or probiotics, omega-3 fatty acids supplements and multivitamins.
Subjects were excluded from the study if they had any of following: contact lens wear, diabetes, pterygium, pregnancy, history of ocular trauma, pinguecula, history of eye surgery, active infection or inflammation of the eye at the time of the study. An optometrist conducted all clinical assessments of subjects.
Diagnosis of MGD
MGD diagnosis was made based on both gland expressibility and quality of secretion score of 1 or greater in either eye with or without lid margin abnormalities as previously reported.5 Among those with MGD, a subject was considered asymptomatic if the Ocular Surface Disease Index (OSDI) score was less than 13 and symptomatic if the OSDI score was 13 or greater.
Fifty-four patients with MGD, based on the MGD workshop criteria with or without posterior blepharitis between the ages of 17 and 40 years, were included in the study group. Another 158 MGD group served as control. The data were collected in 1 month. This was during the routine mandatory screening for all first-year University of Cape Coast students as part of meeting the medical fitness requirement. Subjects who met the inclusion criteria and were willing to provide written informed consent were recruited into the study. Informed consent was obtained from each subject.
A total sample size of 104 was calculated based on an estimated effect size of 0.11 to detect a Pillai trace of 0.1 for two groups and three response variables at an alpha level 0.05% and 80% power. A minimum of 52 participants in each group will be adequate to detect an effect size 0.11. The assumptions were based on average estimates (from previous clinical studies).19 The data set associated with this paper has been used in previous publications.5 13
All participants completed the OSDI, a short version of the Depression, Anxiety, and Stress Scale (DASS-21) and Dry Eye-related Quality of Life Score (DEQS) questionnaire. However, the clinician performing the clinical assessment was not aware of the questionnaires’ results before the clinical examination.
In this study, three validated questionnaires were used. First, the OSDI was used to assess ocular discomfort symptoms.20 21 The short version of the Depression, Anxiety, and Stress scale (DASS-21) was used to determine depression, anxiety and stress in this sample.22 Finally, the response to each scale question was added up and multiplied by 2 to derive the total score for each subscale.22
The DEQS questionnaire (developed by Santen Pharmaceutical Co, Ltd and Dry Eye Society, Japan) is a 15-item questionnaire comprising a degree of disability scale and frequency of symptom scale.23 The summary score was computed with the following formula: summary score: ([sum of the degree of disability scale scores for all questions answered]× 25) divided by the (total number of questions answered).23
Meibomian gland assessment
The clinical assessments included meibomian gland expression with moderate digital pressure (central eight glands of the lower lid), meibomian gland secretion quality, lid margin thickness, lid margin notching and lid margin telangiectasia. All clinical assessment was made for each individual’s left and right eyes. Meibomian gland expressibility and meibum quality: gland expressibility was achieved by pressing the lid margin with moderate digital pressure to express the central eight glands of the lower lid. The number of glands expressing lipid was observed with the slit-lamp biomicroscope. The grading of the glands was as follows: 0=all glands expressible (normal), 1=three to four glands expressible, 2=one to two glands expressible, 3=no glands expressible.24 The quality of lipids oozed out was evaluated for clarity and viscosity. The quality of the expressed lipids was graded as follows: 0=clear (normal), 1=cloudy, 2=cloudy with particles and 3=inspissated (like gel). The highest score for any expressed glands was taken as the quality score.24
Lid margin findings
Lid margin abnormalities were carefully examined for the lower and upper eyelids with the help of a slit-lamp biomicroscope. Lid margin telangiectasia and hyperaemia were evaluated on a scoring scale from 0 to 3: 0=no lid margin redness and no telangiectasia crossing meibomian gland orifices, 1=lid margin redness and no telangiectasia crossing meibomian gland orifices, 2=telangiectasia crossing meibomian gland orifices with a distribution of less than half of the entire length of the lid and 3=telangiectasia crossing meibomian gland orifices with a distribution of half or more of the entire length of the lid.25
Lid margin thickness: 0=no lid margin thickening, 1=lid margin thickening with or without focal rounding and 2=lid margin thickening with complete or diffuse rounding.25
Lid margin notching: 0=no notching is observed, 1=shallow dimpling of the lid margin and 2=deep dimpling of the lid margin.25
Ocular surface assessments
Tear breakup time was measured using a moistened fluorescein-impregnated paper strip. The participant was asked to blink at least twice and then look straight ahead without blinking. The time in seconds from the last blink to the first appearance of a dry spot or dark spot was noted three times with a stopwatch. The average of three readings was recorded as tear breakup time.26
Ocular surface staining was assessed with the Oxford grading scale (0–15), grading the cornea and conjunctiva. This grading was conducted using a cobalt blue-filtered light through a yellow filter to allow conjunctival staining to be visualised.19
The Dry Eye WorkshopII report criteria were used to establish dry eye disease diagnosis, which requires significant dry eye symptoms and at least one positive result of the markers of homeostasis comprising tear osmolarity, tear breakup time and ocular surface staining required to establish diagnosis. Hence, in this study, dry eye diagnosis was made if the OSDI score was ≥13 and there was either ocular surface staining (Oxford grading scale) ≥3 and/or the tear breakup time was <10 s.26
Data analysis
Using SPSS V.21.0 (SPSS Inc) statistical package, all statistical analyses were performed. First, the sample was divided into two main groups based on the presence or absence of MGD. The MGD group was further divided into asymptomatic and symptomatic MGD. t-Tests were used to determine the mean difference between the subjects of the DEQS score and depression, anxiety, and stress scale (DASS-21) subsection scores between meibomian gland dysfunction and non–meibomian gland dysfunction. Since the DASS-21 subsections are strongly correlated, a MANOVA was done before the individual t-test to protect against a type 1 error. For a 95% confidence level, p≤0.05 was considered statistically significant. In order to ascertain whether symptoms or MGD was the main instigator of alteration of quality of life, fixed effects one-way ANOVA was done followed by least significant difference post hoc testing adjusted for the presence of dry eye. The Strengthening the Reporting of Observational Studies in Epidemiology cross-sectional reporting guidelines were used.27
Patient and public involvement
There was no public involvement; however, subjects were engaged individually, and the study’s rationale was duly explained. They did not participate in the conduct of the study, design of the protocol and data collection tools, reporting of the results and dissemination of the study’s findings.
Results
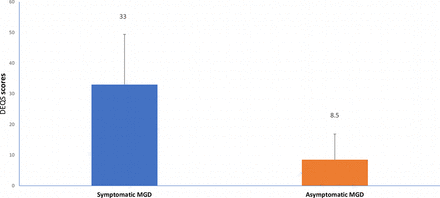
The mean age of the entire sample was 21.9 (±3.8) years, with a range of 17–40 years. The number of males and females in the sample was 105 and 107, respectively. Fifty-four participants had MGD and 158 did not have MGD served as controls. Among the MGD group 33 had symptomatic MGD and 21 had asymptomatic MGD. There was no statistically significant difference in the mean quality of life scores between subjects with MGD and subjects without MGD (t=1.57, p=0.12). The quality of life scores (DEQS) (p=0.022) were significantly higher in the symptomatic MGD group compared with the asymptomatic MGD group. There was no significant difference in quality of life scores (DEQS) (p=0.037) in the asymptomatic MGD group compared with healthy controls. This is shown in table 1.
Difference in means of DEQS scores between MGD groups
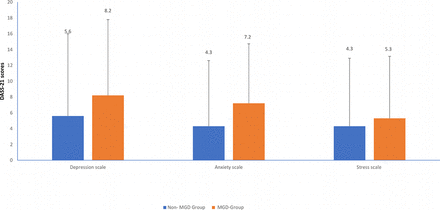
Using Pillai’s trace in the MANOVA, there was a significant effect of MGD on depression, anxiety and stress (V=0.05, F(3,208)=3.76, p=0.012). Separate t-tests were done for each of the subsections of the DASS-21. The depression (p=0.031) and anxiety (p=0.003) subscales showed a significant difference between the MGD group and the non-MGD group; however, there was no difference in the stress (p=0.33) subscale between the groups. Again, using Pillai’s trace in the MANOVA, there was a significant effect of the type of MGD (symptomatic or asymptomatic) on depression, anxiety and stress (V=0.24, F(3, 51)=5.24, p=0.003). The depression (p=0.001) and anxiety (p=0.02) subscales showed a significant difference between the symptomatic MGD and non-MGD groups. However, there was no difference in the stress subscale between the groups. This is shown in table 2.
Mean difference of DASS-21 subsections among MGD groups
Of importance, depression and anxiety subsection scores of the DASS-21 and DEQS scores showed relatively low correlations between themselves and the clinical parameters of MGD with significant correlations coefficient for following: DEQS and meibomian gland expressibility scores (r=0.14 p=0.048); anxiety score and meibomian gland expressibility scores (r=0.17, p=0.012), DEQS and telangiectasia (r=0.16 p=0.021); and DEQS and anxiety (r=0.15, p=0.012). The correlation analysis is shown table 3.
Correlation between MGD parameters, DEQS scores and DASS-21 subsections
The means and SD of various parameters are shown in figures 1–4.
This figure shows means of depression, anxiety and stress scale scores in asymptomatic and symptomatic meibomian gland dysfunction.
This figure shows the mean depression, anxiety and stress scale scores in meibomian gland dysfunction and healthy controls.
This figure shows the mean quality of life scores in asymptomatic and symptomatic meibomian gland dysfunction.
This figure shows the quality of life scores between MGD and non-MGD groups. MGD, meibomian gland dysfunction.
Discussion
MGD is a highly prevalent ophthalmic condition associated with significant ocular sequelae, and it is a considerable threat to ocular surface integrity, homeostasis and comfort.12 A recent meta-analysis estimated the global prevalence of MGD to be approximately 35.8%.28 In Africa, the estimated prevalence is even higher as shown by another meta-analysis reporting a prevalence of MGD around 45.9%.29 In addition, studies conducted in a younger population showed a significant burden of MGD with an estimated prevalence of 25% among the younger population.5 However, studies have not specifically looked at the impact of MGD on quality of life and mental health. This study showed no statistically significant difference in the quality of life scores between subjects with MGD and subjects without MGD.
Furthermore, comparing symptomatic MGD to asymptomatic MGD, it is observed that symptomatic MGD had a poorer quality of life. This implies that amelioration of symptoms in MGD may be more critical in estimating treatment success and effectiveness from the patient’s perspective than the mere improvements in objective clinical signs observed by the clinician.30 31 From the patient’s perspective, treatment for a condition that does not show apparent changes in ocular comfort and quality of life is likely to be lightly esteemed and underappreciated.32 It implies that clinicians treating asymptomatic MGD should carefully educate their patients. The individual patient should be aware that treatment is geared towards arresting anatomical changes to prevent future pathological sequelae that may result in poor ocular surface health. This will enable the patient to manage their expectations and set the tone for an effective doctor–patient relationship.
There was an association between MGD and psychosomatic symptoms, including anxiety and depression but not stress. The exact explanation for these findings is unknown as there appear to be even worse psychosomatic symptoms in symptomatic MGD than in asymptomatic MGD. This conundrum may be partly explained by the fact that ocular surface discomfort shares aetiological and correlational relationships with psychological and chronic pain syndromes.33 Chronic depression may instigate ocular discomfort by enhancing the production and release of proinflammatory cytokines34 that may perpetuate chronic inflammation throughout the body and, more so, the vicious cycle of clinically apparent inflammation seen in MGD.
Some of the correlations between clinical measures of MGD, quality of life scores and psychosomatic symptoms are interesting and worth mentioning. Even though stress and depression were not associated with the clinical measures of MGD, there appeared to be a low but significant association between anxiety levels and meibomian gland expressibility. Again, lid margin telangiectasia was also associated with anxiety levels in this study, consistent with Chiang et al,35 showing increasing anxiety in patients with blepharitis. The exact reason for these potential associations is unknown; nevertheless, in the case of females, one may undoubtedly argue that due to make-up artistry and usage of mirrors, some may occasionally see the unsightly and inflamed lid margins and occasionally make them anxious. Furthermore, the observed loss of eyelashes in severe lid margin disease may explain the increased anxiety observed in patients with MGD and lid margin abnormalities.36 Both meibomian gland expressibility and lid margin telangiectasia are significantly associated with quality of life scores, which may negatively impact patients’ quality of life. Meibomitis often induces ocular surface inflammation such as superficial punctate keratitis, corneal cellular infiltrates and occasionally conjunctivitis.12 Clinicians often fail to differentiate superficial punctate keratitis instigated by dry eye disease from that caused by meibomitis.12 When meibomitis is not apparent to clinicians and the clinician is aware of only superficial punctate keratitis on the cornea, they often institute treatment for superficial punctate keratitis as if it is caused by dry eye disease using dry eye specific eye-drops or other conservative therapy, which may be ineffective. This lead to patients’ frustration and makes them anxious about what is responsible for the ocular surface discomfort. Anxiety may lead patients to run from practitioner to practitioner, which is common among patients with poorly treated ocular surface discomfort due to lid margin disease and MGD.
The findings of this study should be interpreted within context as the cross-sectional study design is limited in predicting causal relationships. The clinical sample used in this study were relatively young, and findings may not be applicable to the aged population.
Conclusion
In conclusion, the study revealed no difference in quality of life scores between MGD and non-MGD groups. However, the symptomatic MGD group had worse quality of life and psychosomatic symptoms than the asymptomatic MGD group and non-MGD group.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
The Institutional Review Board approved the University of Cape Coast study with ethical clearance number UCCIRB/CHAS/2017/04. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors are grateful to Esther Duncan for proof reading the manuscript.
References
Footnotes
Contributors All authors contributed to the conception and the design of the study. SD and SK were involved in acquisition of data. KA analysed and interpretated the data for the study; KA wrote the manuscript. SK act as a gurantor as he has access to all the data sheet. All authors were involved in critical review of the manuscript for important intellectual content. All authors approved the final version of the manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that queries related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.