Article Text
Abstract
Introduction A disturbance in exercise-induced hypoalgesia (EIH) has been observed in patients with chronic whiplash-associated disorders (WAD). Yet, no studies have examined whether EIH occurs in people with acute/subacute WAD. This study will determine whether EIH occurs immediately after and 24 hours after aerobic exercise (AE) and neck-specific exercise (NSE) in people with acute/subacute WAD.
Methods and analysis A randomised controlled trial has been designed and is reported in line with the Standard Protocol Items: Recommendations for Interventional Trials. EIH will be assessed immediately after and 24 hours after AE, NSE and a control intervention (randomly allocated). As dependent variables of the study, we will measure pressure pain thresholds measured over the region of the spinous process of C2 and C5, the muscle belly of the tibialis anterior and over the three main peripheral nerve trunks, Neck Pain Intensity, Neck-Disability Index, Pain Catastrophizing Scale, Tampa Scale Kinesiophobia-11, self-reported Leeds Assessment of Neuropathic Symptoms and Signs Scale.
Ethics approval and dissemination Ethical approval has been granted by the Ethics Committee from University Rey Juan Carlos (Madrid, Spain; reference number 0707202116721). The results of this study will be disseminated through presentations at scientific conferences and publication in scientific journals.
Trial registration number RBR-9tqr2jt, https://ensaiosclinicos.gov.br/observador/submissao/sumario/11551.
- PAIN MANAGEMENT
- Rehabilitation medicine
- Musculoskeletal disorders
- Spine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This trial will evaluate exercise-induced hypoalgesia (EIH) in response to different exercises in patients who have suffered a whiplash injury.
EIH will be assessed as a change in pressure pain thresholds.
This study will assess EIH immediately and 24 hours after the intervention in people with whiplash-associated disorders (WAD).
The influence of psychological variables and neuropathic pain features on EIH will be assessed.
Only people classified as WAD grade II will be included in the study, which could become a limitation to extrapolate the results to all patients suffering with WAD.
Introduction
Whiplash-associated disorders (WAD) is the term given to describe a wide variety of symptoms commonly reported following a whiplash injury.1 After a whiplash injury, most individuals recover within 2–3 weeks; however, up to 42% will suffer persistent pain, resulting in the substantial economical and societal costs.2
It is accepted that an initial peripheral injury could be a source of nociception following a whiplash injury,3 and different structures can be a source of nociceptive pain such as facet joints, intervertebral discs or muscles, among others.4 However, identifying a specific pathoanatomical cause of a patient’s pain following a whiplash injury is often difficult to achieve.5 In addition to nociceptive pain, people with WAD can present with disturbances in the central processing of pain (ie, central sensitisation), neuropathic pain features and the presence of psychological factors.6–8
Exercise-induced hypoalgesia (EIH) refers to a reduction in pain sensitivity following exercise9 due to the activation of endogenous pain inhibitory processes. There are inconclusive results on which is the most appropriate form of exercise, for example, aerobic versus isometric exercise, to reduce pain sensitivity in people with chronic WAD.9 10 Importantly, previous studies have shown that patients with chronic WAD may present with dysfunctional pain inhibition11–13 and, specifically, impaired EIH. Exercise is used early following a whiplash injury with the aim of providing pain relief,14 yet no study has investigated whether EIH can be achieved in people with acute/subacute WAD and what exercise is best to achieve this.
The purpose of this study is to assess whether EIH occurs immediately after and 24 hours after two different types of exercise performed by people with acute/subacute WAD. EIH will be assessed as the change in pressure pain threshold (PPT) at both local and remote sites as a measure of pain sensitivity.15 16 Additionally, we will assess whether the extent of EIH is associated with a reduction in subjective reports of neck pain intensity immediately after and 24 hours after the exercise. As a final aim, we will evaluate whether baseline measures of neck pain intensity, disability and psychosocial factors determine the extent of EIH following exercise in people with acute/subacute WAD. We hypothesise that some patients with acute/subacute WAD will demonstrate impaired EIH following both aerobic exercise (AE) and neck-specific exercises (NSE), both immediately after and 24 hours after exercise; we expect that this impairment will be related to a greater presence of psychological and neuropathic features. Additionally, we predict that the change in pain sensitivity following exercise will be directly related to the extent of reduction in their subjective report of neck pain intensity.
Methods
Trial design
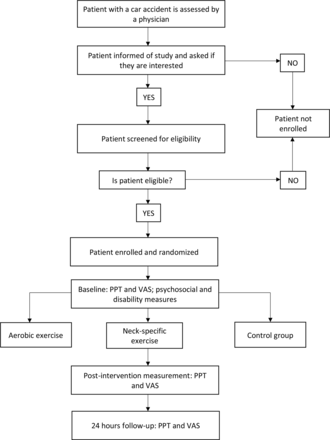
This study is designed as a randomised, controlled, parallel, double-blind, three-arm clinical trial; the study protocol has been designed following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)17 and is registered in a clinical trial registry (https://ensaiosclinicos.gov.br/rg/RBR-9tqr2jt). Participants will be randomised to receive either AE, NSE or a control intervention of passive therapies. The information sheet will not describe the details of the three interventions, and therefore the participant will not be aware of the other interventions. The flow diagram of the selection procedure, interventions and assessments is provided in figure 1, and a populated SPIRIT checklist is provided in online supplemental file 1.
Supplemental material
Subject recruitment and flow through the study. PPT, pressure pain threshold; VAS, Visual Analogue Scale.
Setting
The study will be conducted in the Physical Therapy Department of an outpatient Traumatology Clinic in Madrid, Spain. Patients are referred to this clinic after having a car accident and are evaluated by a physician. If physical therapy treatment is recommended by the physician, then the patient is referred to the Physical Therapy Department, where they are managed by physical therapists with expertise in Orthopaedic Manual Therapy. Before starting the study, the evaluator will be trained in the different assessment procedures to standardise the evaluation.
Participants
All eligible patients consecutively presenting to the clinic with a whiplash injury following a car accident will be approached for recruitment until the sample size is achieved. The physician will determine the grade of WAD according to the Quebec Task Force18 and will determine whether the patient meets the eligibility criteria. If so, the physician will explain the study to the patient and will provide them with the patient information form and if the patient is willing to participate, written informed consent will be obtained.
Eligibility criteria
Inclusion criteria are aged between 18 and 65 years,11 have sustained a whiplash injury within the last 7–30 days, diagnosis of WAD grade II according to QTF and not yet recovered from neck pain at the time of the assessment. Exclusion criteria are WAD grade I, III or IV injury (neurological deficit, fracture or dislocation),11 presence of previous generalised pain or neuropathic pain condition, nerve root compromise (at least 2 of the following signs: weakness/reflex changes/sensory loss associated with the same spinal nerve),9 loss of consciousness after the accident,16 instability signs,19 psychiatric disorders,20 inflammatory or rheumatic disease, or tumours,21 previous surgery in the cervical or upper limbs region,22 previous whiplash injury,16 unwilling to perform a prescribed exercise intervention.11
Randomisation
After providing informed consent, each patient will be randomly assigned to the AE group, NSE group or control group (CG) based on a random sequence (https://www.randomizer.org/). The randomisation sequence will only be known by the principal investigator and auditor.
Blinding
The evaluator and participants in the study will be blinded during the entire process. Participants will not know the description of the other exercise intervention or control intervention. The evaluator will not know which group participants are assigned to. To achieve this, the evaluator will assess the participant, and then leave the room as the participant performs the intervention with another investigator and, when finished, the evaluator will re-enter the room to re-evaluate the participant, approximately 2 min after completion of the intervention. Blinding will be maintained during the 24-hour postintervention assessment.
Sample size calculation
The sample size was calculated using the Grammo calculator V.7.12. Based on the analysis of the variance of means and estimating an alpha risk of 5% (0.05), a beta risk of 20% (0.2), a bilateral contrast, an SD of 15% (0.15), a minimum difference to detect of 15% (0.15), which is based as the minimum clinically important differences on PPT, and a rate of follow-up losses of 10%, 24 participants are required in each group. Thus, we will include 72 patients who will be divided into the 3 groups.
Intervention
Participants will be asked to only perform the assigned exercise intervention; any interference with the prescribed treatment will lead to exclusion. Participants will be asked to avoid analgesic drug intake 24 hours prior to the intervention and reassessment,9 caffeine intake 8 hours before the intervention9 and to avoid physical activity other than daily activities, 24 hours before the intervention and reassessment.9 The reassessment will take place at the same time of day as the first session. The intervention will take place in a Traumatology Clinic; patients will be managed by one of two physical therapists. Both therapists (ML-A and EP-V) have expertise in Orthopaedic Manual Therapy with at least 2 years of experience, and they will be trained to deliver the intervention by EA-L.
Aerobic exercise
A submaximal AE intervention will be performed using a cycle ergometer (Kardiomed 520 basic cycle, Proxomed, Alzenau, Germany). The seat will be adjusted to suit each participant. The exercise protocol is based on the Aerobic Power Index Test,23 previously used in similar studies.9 24 The duration of the test will be kept below 20 min, thus avoiding early fatigue in the lower extremities.25 The submaximal level is defined as 75% of the age predicted maximal heart rate ((220−age)×0.75). The participant will start at 25 W and approximately at a constant pedalling rate of 60 rpm, will maintain this intensity a minute for warm-up. Then the power output will be increased by 25 W every minute until the participant reaches their individual target heart rate, maintaining this power output for 17 min; then, power output will be reduced to 25W again for cooling down (2 min). Heart rate will be recorded each minute during the increase in power output and then once every 3 min until the end of the exercise session. The total exercise time will be 20 min.
Neck-specific exercise
Two NSE will be implemented. They have been selected since they have either resulted in a reduction in pressure pain sensitivity after exercise,26 a decrease in neck pain intensity or disability following the exercise27 or an improvement in muscle function.28–30 Approximately 5 min will be spent first, teaching the patient how to perform the exercises. Two different exercises will be performed with a short rest in between for a total time of 20 min.
Craniocervical flexion (CCF) exercise
Participants will perform CCF exercise in supine, following on an established protocol.31 32 This task consists of flexion of the cranium over the cervical spine without lifting the head from the supporting surface. The therapist will first determine, using a pressure biofeedback device (Stabilizer; Chattanooga Group, Chattanooga, Tennessee, USA), the highest pressure increment (from 22 to 30 mm Hg)33 the participant can correctly sustain for 10 s. Once this is determined, they will perform 3 sets of 10 repetitions of 10 s duration, at this target level with a 10 s rest interval between each contraction and 1 min rest interval between sets (total contraction time=300 s, total time of exercise=690 s).
Cervical extension (CE) exercise
Participants will be asked to position themselves in four-point kneeling, and a mid-resistance elastic band (Pilates Band Medium, Decathlon, Villeneuve d’Ascq, France) will be placed over their head, as they hold the elastic band with their hands. The participant will be required to perform CE with the cervical spine in a neutral position against the resistance of the elastic band. During the first 5 min of the session, each participant’s pain-free 12 repetition maximum will be assessed. If the participant can perform 12 repetitions with no pain, this will be the exercise performed. If they are unable to perform 12 repetitions, the elastic band will be changed to one of lower resistance (Pilates Band Light, Decathlon). If the participant is still not able to perform the exercise, it will be performed without an elastic band or they will be moved to a position of prone on elbows. Three sets of ten repetitions at the predetermined intensity level will be performed with each repetition lasting 3 s, with 3 s of rest between repetitions, and 30 s between sets (total contraction time=90 s, total time of session=231 s).
Control intervention
The CG will receive an intervention considered as a placebo, based on a previous study.27 First, ultrasound therapy will be applied over the trapezius muscle bilaterally, with the patient in prone. The ultrasound will be applied for 4 min over each side, with 30 s rest between sides. Following a further 30 s of rest, laser therapy will be applicated over the C2/C3 level, for 5 min. Following a further 60 s of rest, the patient will be positioned in supine and the therapist will place their hands without therapeutic intention on the patient’s neck for 5 min. The total duration of the session will be 20 min.
Outcome measures
Pressure pain threshold
The PPT will be the primary outcome measure to quantify EIH and will be recorded in Newton/cm2 using a digital algometer (Force TenTM -Model FDX; Wagner, Greenwich, Connecticut, USA) with a round tip surface area of 1 cm2. The measurements will be taken over several sites in the following order: (1) the spinous process of C2 and C5, providing a measure of local pain sensitivity; (2) muscle belly of the left tibialis anterior, providing a measure of remote sensitivity; and (3) three bilateral upper limb sites (over the three main peripheral nerve trunks). These sites have already been used in investigations of pain sensitivity in patients with WAD.11 15 The evaluator will gradually increase the pressure until the patient indicates ‘yes’ at the first perception of pain. Two measurements will be taken at each site, with 30 s between each measurement, obtaining an average of the PPT at each site for the statistical analysis.25 This measure will be taken at baseline, post intervention and 24 hours later. Relative EIH will be defined as a significant positive change in PPTs, that is, when PPT increases after exercise, according to the following formula: ((PPT post exercise–PPT pre exercise)/PPT pre exercise))×100.
Self-reported pain intensity
Self-reported neck pain intensity will be measured using a Visual Analogue Scale. Participants will be instructed to indicate their current pain intensity by drawing a vertical line on a 0–100 mm horizontal line, with 0 representing no pain and 100 unbearable pain, obtaining a score ranging from 0 to 100. This outcome has good validity and reliability.34 This outcome will be measured at baseline, immediately post exercise and 24 hours post exercise.25 Pain intensity will be evaluated always just before PPT assessment.
Additional patient reported outcome measures assessed only at baseline
Neck Disability Index (NDI)
The NDI is a self-assessment instrument of the specific functional status of subjects with neck pain. It consists of 10 items, each of them rated on a 6-point scale with responses ranging from no disability (0) to complete disability (5). An overall score is generated by summing the score for each item and multiplying by 2. The NDI has been widely applied in patients with WAD with good reliability and validity, and has been validated in Spanish.35 36
Pain Catastrophizing Scale (PCS)
PCS is a self-administered scale consisting of 13 items on catastrophic thinking about pain. All items are rated in a 5-point. The total score is generated by summing the ratings of each item.37 PCS has been used in patients with WAD and is validated in Spanish.38 39
Tampa Scale Kinesiophobia-11 (TSK-11)
TSK-11 is a self-administered questionnaire consisting of 11 items designed to assess fear of movement/(re)injury in which patients are instructed to rate each item on a 4-point scale.40 This scale has been used in patients with WAD and translated to Spanish.41 42
Self-reported Leeds Assessment of Neuropathic Symptoms and Signs Scale (S-LANSS)
This is a self-report version of the LANSS Scale.43 It is composed of 7 items and includes 2 self-examination items. A score of 12 or greater identify patients with pain of a predominantly neuropathic nature. It has been used in patients with WAD16 and validated to Spanish.44
Chronic Disease Self-Efficacy
The Spanish version of this scale will be used.45 This scale has already been used in patients with acute/subacute WAD and consists of four items whose ranges from 0 ‘very insecure’ to 10 ‘very safe’. The total score ranges from 0 to 40, with higher scores reflecting greater self-efficacy beliefs.46
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Statistical analysis
An intention-to-treat analysis will be carried out using IBM-SPSS Statistics V.24 software. The normality test applied to all the variables will be the Kolmogorov-Smirnov test. For the contrast of intragroup hypotheses, both in the short term and 24 hours after the intervention, Student’s t-test for paired variables will be applied in the case of parametric distributions and Kruskal-Wallis H for non-parametric distributions. Effect size will be calculated through eta squared; values of r2 will be considered as 0.01 (small), 0.06 (medium) and 0.14 (large). To compare the extent of EIH between groups, both in the short term and 24 hours after the intervention, one-factor analysis of variance will be used in the case of parametric distributions and Kruskal-Wallis H for non-parametric distributions. Post analysis will be obtained through Bonferroni’s contrast for parametric distributions and Mann-Whitney’s U for non-parametric ones. Associations between the extent of EIH and other variables will be analysed via regression analysis. The confidence level used will be 95% (0.05), and the power of the study will be 90% (0.1).
Discussion
This protocol paper describes a randomised controlled trial which will determine whether EIH, measured as a change in PPT, occurs in patients with acute/subacute WAD in response to two different exercises and whether EIH is sustained 24 hours later.
Exercise is a fundamental intervention for physical therapists to prescribe for the management of musculoskeletal pain, including for patients with WAD.47 By examining the effects on pain sensitivity following either AE or NSE, we will be able to determine whether either exercise approach can be used to induce immediate pain relief for patients with acute/subacute WAD. We may find that, comparable to patients with chronic WAD,9 13 some people with acute/subacute pain following a whiplash injury do not respond favourably to the exercises, especially since these patients may have increased pain sensitivity.15 20
Our results also intend to establish whether the extent of EIH following exercise is determined by other factors including their level of pain and the presence of psychological factors. A recent study found that self-efficacy beliefs are an important factor in patients with acute/subacute WAD, and that kinesiophobia mediates the association between self-efficacy and pain catastrophising.44 In the current study, we will examine whether the extent of such features affect the EIH response. Given that a neuropathic component may explain the clinical presentation of some patients with acute pain following a whiplash injury,16 we will also examine the relationship between neuropathic features and the extent of EIH.
Trial status
This is the first version of the study protocol. Participants will be recruited between February 2022 and December 2022. Study completion is expected to be May 2024.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Deb_Falla
Contributors CRB is the director of the project, contributed to the protocol development, provided clinical expertise and is responsible of designing the statistical procedures. DF is the codirector of the project, contributed to protocol development and methodological considerations, and provided clinical expertise. ML-A and EP-V are the two physical therapists who performed the interventions for the study. FJR-D-R helped in the organisation of subjects and data extraction. EA-L and CB-U are the main investigators who run the study; they contributed to the concept and study design, provided clinical expertise and developed the manuscript with feedback from all authors. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.