Article Text
Abstract
Objectives To evaluate the feasibility and outcomes of an occupational therapy lifestyle intervention for adults living with chronic pain.
Design This one-group pre-post interventional study investigated the feasibility and outcomes of the Redesign Your Everyday Activities and Lifestyle with Occupational Therapy (REVEAL(OT)) intervention targeting meaningful activities and lifestyle.
Settings The occupational therapist-led intervention was added to standard multidisciplinary chronic pain treatment at a Danish pain centre.
Participants Of the 40 adult participants aged 18–64 (mean 46.6±10.9 years old, 85% females, chronic pain duration ≥3 months), there were 31 completers.
Intervention Three feasibility rounds were carried out in 2019–2021. The intervention focused on meaningful activities, healthy eating habits and daily physical activity. Methods of didactical presentations, group discussions, personal reflection and experiential learning were used in the intervention composed both of individual and group sessions.
Outcomes Primary outcomes were predefined research progression criteria evaluated by the red-amber-green method. Secondary outcomes measured pre-post changes in health-related quality of life and occupational performance and satisfaction.
Results The study demonstrated satisfactory programme adherence (77.5%), patients’ self-perceived relevance (97%), timing and mode of delivery (97%) and assessment procedure acceptance (95%). No adverse events causing discontinuation occurred. Recruitment rate (n=5.7 monthly), retention (77.5%) and the fidelity of delivery (83.3%) needed improvement. We observed no improvement in health-related quality of life (mean=0.04, 95% CI −0.03 to 0.12) but positive change in occupational performance (mean=1.80, 95% CI 1.25 to 2.35) and satisfaction (mean=1.95, 95% CI 1.06 to 2.84). The participants reached the minimal clinically important difference for occupational performance (≥3.0 points in 13.8%) and satisfaction (≥3.2 points in 24.0%).
Conclusions The REVEAL(OT) intervention was feasible to deliver and beneficial for the participants’ occupational performance and satisfaction. The interventions’ recruitment, retention and delivery strategies need optimisation in a future definitive trial.
Trial registration number NCT03903900
- PAIN MANAGEMENT
- REHABILITATION MEDICINE
- PUBLIC HEALTH
- COMPLEMENTARY MEDICINE
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first study to evaluate the feasibility of a novel occupational therapy lifestyle intervention added to the standard multidisciplinary chronic pain treatment at a Danish pain centre.
The one-arm single-centre design and a small sample size of this study limited its external validity.
Despite encouraging change in occupational performance and satisfaction, no blinding in the assessment procedure may have introduced detection bias.
Lack of evaluation of the contextual feasibility did not fully allow to clarify the intervention’s potential as an add-on treatment.
Introduction
Multidisciplinary and multimodal treatment is the most beneficial approach to improve the quality of life in people living with chronic non-malignant pain,1 defined as pain lasting longer than 3 months and exceeding the expected recovery time.2 Previous research has urged new non-pharmacological treatment modalities such as lifestyle management in chronic pain treatment.3–5 A recent survey revealed multiple elevated lifestyle-related health risks (n≥2), such as body mass index ≥30, sedentary lifestyle, unhealthy eating habits, poor physical fitness, low sleep quality, high stress level and cigarette smoking, in 58% of the adults living with chronic pain referred to chronic pain rehabilitation.6 Along with the findings, moderate to high motivation for improving lifestyle observed in this population supported the relevance of focusing on lifestyle in patients with chronic pain.6
Occupational therapy has a decade-long history of targeting lifestyle through everyday occupations, improving human health and well-being.7–10 A recent pilot study using the occupational therapy Lifestyle Redesign programme in a Canadian setting showed significant improvements in occupational engagement, life balance, mental health and pain self-efficacy in patients with fibromyalgia.11 However, more research on occupational therapy lifestyle management for chronic pain is still needed.12
We developed an occupational therapy lifestyle management programme Redesign Your Everyday Activities and Lifestyle with Occupational Therapy (REVEAL(OT)) for adults living with chronic pain and adopted it to the standard multidisciplinary treatment of chronic pain offered at a Danish hospital. According to the Medical Research Council (MRC) framework for developing and evaluating complex interventions, novel treatment programmes need comprehensive feasibility evaluation before initiating a full-scale randomised controlled trial (RCT).13 The objectives of the present study were to evaluate the feasibility and outcomes of the REVEAL(OT) intervention and determine further research steps before initiating an RCT.
Methods
Study design
This one-arm pre-post prospective feasibility study followed the same protocol as expected for the RCT, excluding randomisation (ClinicalTrials.gov registration number: NCT03903900, registration date: 4 April 2019). The study comprised three feasibility rounds of the REVEAL(OT) 1.0–3.0 that took place between April 2019 and July 2021. Guided by the MRC framework,13 the iterative feasibility testing process should help improve the intervention and prepare it for the future RCT. The research complied with the principles of the World Medical Association’s Declaration of Helsinki, the European Union’s General Data Protection Regulation and the Danish Data Protection Act.14 15 The participants were required to sign an informed consent before participating. This report followed the Consolidated Standards of Reporting Trials guidelines for reporting non-randomised pilot and feasibility studies (online supplemental appendix 1).16
Supplemental material
Settings
Two clinical units at Næstved Hospital in Region Zealand, Denmark, were involved in the chronic pain treatment delivery. The Multidisciplinary Pain Centre (MPC) delivered standard treatment based on cognitive–behavioural therapy provided by physicians, nurses, psychologists, physiotherapists and a social worker. The Occupational Therapy Unit (OTU) delivered the REVEAL(OT) intervention.
Participants
Adults aged ≥18 to ≤64 years referred to the MPC with chronic non-malignant pain present ≥3 months at the inclusion were invited to participate. Exclusion criteria were:
Diagnoses of acute/subacute pain, cancer-related pain, headache/migraine, depression (current), substance misuse (current) and severe psychiatric diagnosis (eg, schizophrenia and schizotypal, delusional, schizoaffective or psychotic disorders, or psychosis).
Medicine intake increasing 30 mg of morphine equivalent daily or irregular medication pattern over the past 4 weeks.
Poor Danish-speaking skills.
Participation in other chronic pain treatment programmes.
Inability to walk a distance of a minimum 100 m independently (added to secure the study cohort homogeneity).
Well-treated headaches, antidepressants against depression relapse or similar conditions in medical history were allowed if not the primary cause for the MPC referral. Habitual (not newly entered) physical training was neither an indicator for exclusion.
Recruitment
The MPC team screened the outpatients for age and interest in participation, referring the relevant candidates to the OTU. The principal investigator provided detailed written and oral information on participation and performed eligibility screening. At least 1 week of consideration was provided, including additional phone or email contacts when relevant.
Intervention
The intervention description complied with the Template for Intervention Description and Replication recommendations (online supplemental appendix 2).17
Supplemental material
The REVEAL(OT) focused on value-based choice of meaningful activities and lifestyle choices, regarding healthy eating and daily physical activity. By focusing on value-based choice of meaningful activities, the REVEAL(OT) pursued to activate the transformative capacities of human occupation as an interaction between the occupational dimensions Doing, Being, Becoming and Belonging.18 19 Physical activity guidelines for adults from the WHO20 and healthy nutrition advice from the Danish population from the Ministry of Food, Agriculture and Fisheries in Denmark21 in their versions available in 2019–2020 supported practising healthy lifestyle choices. Furthermore, the REVEAL(OT) construct was inspired by the Lifestyle Redesign programme, adapting its approach to meaningful activities and a healthy lifestyle, combining individual and group sessions and using the methods of didactic presentation, peer exchange, personal reflection and direct experience.22 Each group could admit a maximum of six patients.
The REVEAL(OT) 1.0–3.0 consisted of two to four individual sessions of 1 hour and four to eight group sessions of 2 hours over 12–15 weeks (at n=2 individual sessions per programme, up to seven phone-based or video-based individual contacts were provided). At baseline, the patients identified their occupational problems related to productivity, self-care and leisure activities that inspired further goal setting. Besides the assessments at baseline and follow-up, session topics covered: introduction to the course, occupation for health and well-being, benefits of daily physical activity, meals and eating habits, occupational balance and time management, productivity/domestic activities (in home), productivity/activities out of home, ergonomics, flow experience, hobbies and leisure, goal setting, goal evaluation, home visits and ending the group. Lifestyle diaries and pedometer for step counting supported the maintenance of the initiated lifestyle changes at home. Assistive devices for home use to support working with individual occupational goals were available for borrowing.
Two occupational therapists (OTs) who provided the intervention had 14 years of professional experience each. The OTs and the principal investigator attended the online continuing education course ‘Life Management Series: Lifestyle Redesign for Chronic Pain and Headache Management’ approved by the American Occupational Therapy Association. The therapists were provided with supervision by the principal researcher at least once a week or on demand. The programme featured contacts with OTs at least every second week.
The REVEAL(OT) was manualised and protocolised. The MPC team was contacted to solve additional patient-related issues when relevant. On the intervention discharge, the patients continued their planned standard treatment at the MPC.
We described the REVEAL(OT) in details in online supplemental appendix 3 by applying the occupational therapy intervention taxonomy based on the Person-Environment-Occupation model23 and proposed by the latest review on occupational therapy for chronic pain12 to the manualised intervention contents. The structural adjustments in the intervention were made to meet the actual needs in the study cohort and clinical practice, while the threefold focus and topics included remained. Regardless of the adjustments, all the participants followed the same procedures.
Supplemental material
Patient and public involvement
Feasibility outcomes in this study investigated the priorities, experiences and preferences of patients with chronic pain through systematic evaluations, which informed the alterations between the feasibility rounds. Treatment-related outcomes in this study were inspired by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations on core outcomes for chronic pain trials expressing the needs of the global chronic pain population in monitoring pain, physical and emotional functioning and sleep, which altogether may affect their health-related quality of life (HRQoL).24 25 Besides the international evidence, the outcomes were informed by an exploratory survey on HRQoL, health, pain, lifestyle factors and motivation for changing lifestyle in adult patients with chronic pain referred to the local clinical practice.6 No patient advisers were involved. After this study’s outcomes are described and reported in peer-reviewed publications, a cumulative report in Danish will be disseminated to the participants’ digital ID-based mailboxes.
Data collection
Gender, age, years with pain and general health status at baseline were collected through PainData, the national registry for patients with chronic pain referred to pain centres in Denmark.26
Primary outcomes
Primary outcomes were predefined research progression criteria based on the ‘traffic light’ (red-amber-green) method (table 1).27
Research progression criteria for feasibility outcomes
Supplemental material
Recruitment was registered by personal project ID and date for eligibility screening, inclusive notes on allocation, withdrawal reasons or eligibility. Participant retention and programme adherence were recorded in each group’s attendance forms, with backup in the electronic patient journals and appointment schedule for the OTU. For patients’ self-perceived relevance, timing and mode of delivery, the participants independently completed the patient evaluation forms developed for the intervention. The patient evaluation forms asked the participants:
Was the scheduling of the session appropriate?
Was the timeframe for the session reasonable?
Were the session contents relevant?
Were the intervention contents easy to comprehend?
Was the mode of delivery (individual or in group) appropriate?
Were you satisfied with your participation in the session?
The patient evaluation forms in the REVEAL(OT) 1.0–2.0 were based on a three-item Likert scale (‘Agree’, ‘Disagree’ or ‘Don’t know’), while a six-item Likert scale (‘Fully agree’, ‘Agree’, ‘Neither/nor’, ‘Disagree’, ‘Definitely disagree’ or ‘Don’t know’) was used in the REVEAL(OT) 3.0. In addition, both forms were provided with a comment box.
Fidelity of delivery and assessment procedure acceptance were evaluated using process evaluation forms completed by the OTs after each session. The process evaluation forms bullet listed the session contents described in the intervention manual and allowed a rapid check of actions performed. Notes and comments were discussed during supervision with the principal researcher, and the challenges were solved by additional demonstration and instruction, for example, in performing relevant assessments.
Adverse events were defined as unpleasant experiences such as discomfort, morbidity and mortality causing discontinuation from the REVEAL(OT).28 Adverse events were registered by the intervention providers and monitored in the electronic patient journals, assessing the date of occurrence, duration and potential consequences. Additionally, self-reported adverse events were derived from the PainData registry. Finally, causes for discontinuation from participation were clarified by phone.
Secondary outcomes
The secondary outcomes were assessed within 2 weeks before and after intervention participation. This paper reports on self-reported HRQoL that will be the primary outcome for the RCT and occupational performance and participation as the outcome that guided the goal work during the intervention. All the secondary outcomes evaluated in the feasibility study (online supplemental appendix 4) will be described in detail and reported separately, referring to this paper.
Health-related quality of life
5-Level version of EuroQol-5 Dimension (EQ-5D-5L) questionnaire (EuroQol registration ID: 28126, further EQ-5D) assessed problems in mobility, self-care, usual activities, pain/discomfort and anxiety/depression ranging on a 5-point categorical scale from 1=‘no problems’ to 5=‘extreme problems’ (EQ-5D values) and self-perceived health on a 0–100 points visual analogue scale where 100 was the best imaginable health (EQ-5D VAS).29–31 From the EQ-5D data derived from the PainData registry,26 we calculated the cumulative HRQoL score (EQ-5D Index) using the Danish EQ-5D Crosswalk value set.32 The EQ-5D Index ranging from −0.594 to 1 considers all states below zero being ‘worse than death’, while ‘1’=‘perfect health’.33
Canadian Occupational Performance Measure
The Canadian Occupational Performance Measure (COPM) helped identify and prioritise individual occupational problems related to self-care, productivity and leisure on a 10-point scale according to their self-perceived importance, performance and satisfaction with the performance (higher scores mean higher importance, performance and satisfaction).34 The COPM assessment as an outcome is valid, reliable and sensitive to change, that is, in chronic pain studies.35
Sample size
This study followed the rationale for feasibility studies which typically are smaller exploratory studies, not testing a hypothesis and thus not requiring sample size power calculation.36 37 According to the rationale about feasibility, we considered a minimum of 12 participants sufficient for this feasibility study, including a number or per cent required to reach the boundaries in predefined research criteria.38 Acceptable dropout of a maximum 20% corresponded to the limits determined for the RCT.
Analysis
This feasibility study was designed to perform primarily descriptive analyses and inform a future larger study.39 Primary outcomes were analysed for frequencies of the research criteria fulfilled, using Microsoft Excel software V.16.53, and compared with the predefined satisfactory estimates in the ‘traffic light’ method (table 1). All feasibility outcomes assessed until the completion date of the study (for the completers) or loss to follow-up (for the non-completers) were included in the primary outcome analysis.
The six-item evaluation forms for participants’ self-perceived relevance, timing and mode of delivery were collapsed into three items for better comparability. Hence, the answers ‘Fully agree’ and ‘Agree’ were categorised as ‘Agree’, and ‘Neither/nor’, ‘Not agree’ and ‘Definitely not agree’ as ‘Not agree’. The category for indefinite answers (‘Don’t know’) remained unchanged. The participant comments supported the interpretation of the results. Only adverse events causing discontinuation from the intervention were eligible for evaluation using the predefined research criteria. All adverse events were considered in terms of further intervention improvements. Evaluating the fidelity of delivery, we considered any session with ≥1 action deviating from the intervention protocol or manual for a delivery failure. The delivery failures were analysed for frequency and described using additional comments and observations on possible reasons.
The secondary outcome analysis included all the participants who had baseline and follow-up assessments. Differences in change pre-post intervention in HRQoL and COPM in the intervention completers were assessed using paired t-tests. As the COPM scores do only provide information on pre-post change in person, the recommended cut-offs for the minimal clinically important difference (MCID) for COPM occupational performance (≥3.0 points) and satisfaction (≥3.2 points) expressing the difference in any direction should support the evaluation.40 41 The statistical analyses of secondary outcomes were performed using Stata V.17.0 (Stata Statistical Software: Release 21. College Station, Texas: StataCorp). The 5% significance level guided the interpretation of the outcomes observed.
Results
From January 2019 to October 2020, one hundred and seventy-four outpatients were referred to the OTU regarding participation (figure 1). Several outpatients were either not interested in participation (n=54) or not reachable by phone (n=17) despite a minimum of five attempts per outpatient. Thus, of the 103 outpatients assessed for eligibility, 57 were included. Of those participating from January to March 2020, seventeen participants (three groups) discontinued involuntarily because of the COVID-19 lockdown and were excluded from the primary analysis.
Study flow diagram. REVEAL(OT), Redesign Your Everyday Activities and Lifestyle with Occupational Therapy.
Participant characteristics
The 40 participants (85.0% females) were aged 46.6±10.9 (23–64) years and had average pain duration of 10 years (median: 9.3; range 0.7–39) (table 2). There was no significant difference between the completers and those who discontinued due to the lockdown (age, p=0.80; gender, p=0.75) or those who could not participate (age, p=0.72; gender, p=0.05).
Sociodemographic and health-related characteristics of the study cohort
Feasibility evaluation
The study’s primary outcomes were summarised (table 3) and explained below.
Research progression criteria summary
Recruitment rate
Although the recruitment rate was 4.3–6.7 participants per month (n=5 per group) and seemed satisfactory according to the research criteria in the eight groups that received the programme, some groups ranged lower (two groups with four participants). Thus, the recruitment criteria n≥3 per month did not guarantee a minimum of five participants in all groups. Recruitment reached the green level in REVEAL(OT) 2.0 and 3.0. However, we downgraded our overall evaluation of the research progression criteria for recruitment rate to the amber level.
With the mean of five participants per group and 1 year from baseline to the primary endpoint, one OT could deliver the REVEAL(OT) in its version 3.0 to mean 20 outpatients (four groups) annually or 40 (eight groups) in 1.5 years. Thus, we would need to engage from 6 to 11 research sites in the future RCT estimated to include 228 participants. Of the total number of outpatients referred to the intervention, 30 (17.1%) remained on the waiting list because no vacant place or appropriate group was available. Thus, running two or more groups at a time could be considered if clinical capacities allow.
Participant retention
Excluding the 17 participants who discontinued involuntarily due to COVID-19, the main reasons for dropout (n=9 of 40; 22.5% dropout rate) were mental or cognitive surplus deficit (n=6). Raised emotional pressure under the pandemic because of additional load, for example, caretaker duties and homeschooling for children, or isolation and fear of the disease, used to cause lack of surplus. In total, 31 (77.5%) participants completed the feasibility study, corresponding to the amber level for research progression. In the non-pandemic-exposed groups in the REVEAL(OT) 1.0–2.0, the retention reached 90.9 and 100% regarding in-clinic assessments. Please see the graph for retention in the REVEAL(OT) 1.0–3.0 in online supplemental appendix 5.
Supplemental material
Issues with timely completing the online questionnaires were observed. Of the 31 pre-post questionnaire sets expected to be completed, 23 (74.2%) were returned. Efforts were made to secure the questionnaire completion, with a mean of five attempts per participant, including phone calls, digital post and messages. However, eight (25.8%) participants had missing questionnaire data either at baseline or follow-up, or both, reportedly because of forgetfulness. In general, very few participants initiated a contact themselves to solve challenges with completing the online questionnaires at home. At the same time, no significant challenges were observed in adherence to the assessment in clinic. The participants were offered time and electronic devices to complete the questionnaire in the clinic. Still, few had a surplus to stay longer than the regular assessment session that varied from 2 to 2.5 hours. In the REVEAL(OT) 3.0, the completion rate improved since the principal researcher received administrator access to the online questionnaire and further promoted the completion.
Programme adherence
We calculated the intervention sessions per participant before regular discharge (n=31) or declared discontinuation (n=9). The 40 participants attended 412 (83.9%) sessions of 492 available, mean n=83.5% (38.5–100) sessions attended per participant. Thirty-one (77.5%) participants adhered to 75% or more of the programme sessions delivered, placing the research progression criteria for programme adherence on the green level.
Patients’ self-perceived relevance, timing and mode of delivery
Of the 343 evaluation forms completed, 97.0% (91.9–100) of the participants responded positively to the questions (online supplemental appendix 6), placing the research evaluation criteria on the green level. Some participants proposed schedule revision (5.8%), for example, establishing afternoon groups or providing more time for peer discussions and contact with OTs (4.1%).
Supplemental material
Assessment procedure acceptance
Of the 40 participants, two (5%) refused to participate in the assessments such as the cuff algometry test at follow-up because of discomfort at baseline (n=1) or wearing an actigraphy unit because of prior occurrence of allergy (n=1) despite non-allergic medical tape provided. Thus, the research criteria for assessment procedure acceptance were 95.0%, corresponding to the green level. However, most participants demonstrated various degrees of exhaustion after both assessment rounds.
Adverse events
Adverse events were registered in 8 (20%) participants, for example, depression (n=2), short hospitalisation for observation of heart (n=1) and gut (n=1), hospitalisation (anticipated, no date at baseline) for knee operation (n=1), emergency room visit (n=1), leg pain after cuff algometry (n=1) and consulting a psychiatrist (n=1). None of the events were associated with the intervention. The depression diagnoses were obtained after intervention discharge and did not cause discontinuation. Discontinuation because of knee operation was expected but not scheduled at baseline. No serious adverse events led to the discontinuation of the intervention, placing the research criteria on the green level for adverse events.
Fidelity of delivery
Of the 233 (80.1%) process evaluations completed by the OTs after a session delivered (one per group or participant for group or individual sessions, respectively), 39 (16.7%) contained 45 amendments to the protocolled contents. Of those, 22 (48.9%) were not timely completed online PainData registry questionnaires; 9 (20.0%) indicated extra session time needed; 9 (20.0%) reported changes in testing order of planning convenience reasons; 3 (6.7%) registered difficulties in following the programme due to concentration deficit (all in the participants from the COVID-19 pandemic-exposed groups); and 2 (4.4%) showed declined participation (in cuff algometry and actigraphy testing as described above). All the amendments were related to single actions, not the entire session content. However, if counting a session with any modification for a delivery failure, the fidelity of delivery with 194 entirely correctly performed sessions was 83.3%, placing the research criteria on the amber level. During supervision, the therapists’ feedback revealed high flexibility demands in patient contact, for example, extra calendar space available for alternative appointment times because of many acute amendments to the schedule or other urgent solutions.
Accept of randomisation
Additionally, we asked the participants who entered the REVEAL(OT) 3.0 programme (n=37) whether they would have participated if the study was an RCT, where controls would not receive the occupational therapy intervention. We received 36 (97.3%) positive responses, while one participant declined because of randomisation chance to no intervention.
Patient-reported outcomes
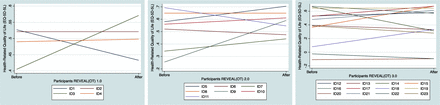
Of the 24 participants with complete pre-post online questionnaires, one was excluded because of deviations from the treatment plan (receiving higher doses of the standard treatment before starting in the REVEAL(OT)). We observed no significant pre-post difference in HRQoL (figure 2 and table 4).
Differences in secondary outcomes pre-post intervention
Participants’ pre-post trajectories in health-related quality of life (EQ-5D-5L, 5-Level version of EuroQol-5 Dimension). REVEAL(OT), Redesign Your Everyday Activities and Lifestyle with Occupational Therapy.
The pre-post difference in COPM (table 4) scores showed an improvement in occupational performance and satisfaction with occupational performance, indicating overall successful and satisfactory resolution of self-reported occupational problems. However, only 13.8% of the participants reached the MCID cut-off of ≥3.0 points for the COPM occupational performance, while 24.1% reached the MCID ≥3.2 points for satisfaction. In the REVEAL(OT) 3.0, those levels were 27.3% and 45.5%, respectively.
Discussion
This study evaluated the feasibility of a lifestyle-oriented occupational therapy intervention REVEAL(OT), added to the standard multidisciplinary treatment of adults living with chronic pain. The predefined research progression criteria regarding programme adherence; patients’ self-perceived relevance, timing and mode of delivery; assessment procedure acceptance; and adverse events were met, indicating that the REVEAL(OT) intervention is feasible. Several challenges regarding recruitment, participant retention and the fidelity of delivery were identified. As the study was not powered to detect differences, the outcomes need to be interpreted with caution. While we observed no change in HRQoL, the COPM scores for occupational performance and satisfaction increased. The proportion of the participants who reached the MCID for the COPM scores increased in the last feasibility round. This study nuanced the patient-reported beneficial effect of the REVEAL(OT) investigated previously by qualitative methodology, for example, increased acceptance of living with chronic pain and empowerment for changing lifestyle.42 The iterative feasibility testing process progressively improved the quality of the REVEAL(OT), particularly regarding patients’ self-perceived relevance, timing, and mode of delivery, and fidelity of delivery. This feasibility study informed further research steps to prepare the evaluation of intervention effectiveness.43
Recruitment and retention are critical in trials and can often be challenging.44 The current clinical capacity at the OTU was limited by a few OTs, not allowing additional intervention therapists involved. Thus, almost every fifth outpatient referred to the REVEAL(OT) remained on the waiting list. Even having those included, we would not reach the sample size estimated for the future RCT. This experience highlights the need for a multicentre study design to secure the timely RCT completion.44
For participants’ retention, further reduction of the assessment load and easier control of self-assessments would be beneficial and prevent missing data, which is crucial for an RCT.45 46 The possibility of conducting the entire assessment session in clinic, where project assistance can provide on-site support, can be considered. However, while revising the test battery, other relevant assessment tools could be considered for inclusion, for example, measuring readiness for change.47 48 According to our earlier qualitative mid-term evaluation, readiness for participation among the REVEAL(OT) patient groups would increase motivation and peer interaction.42 Such information obtained at baseline could also help identify the need for further support of the participants to increase retention.
Long-lasting pain itself imposes a higher risk of vulnerability.49 50 We experienced that the COVID-19 pandemic aggravated the vulnerability in the study cohort, supporting other evidence on the elevated levels of anxiety, isolation and inactivity in people living with chronic pain during this period.51 52 In contrast, the COVID-19 pandemic effect on the study cohort differed from those on office workers, where significantly less pain (in both sexes) and enhanced physical activity (especially in women) were observed after a period of teleworking.53 However, most of our study population was out of the labour market and, therefore, may suffer more difficulties in self-adaptation to changes in the every day. Mental and cognitive load has previously been seen to threaten research participation.54 Consequently, we would propose additional efforts to support retention despite possible social restrictions, for example, more comprehensive online treatment delivery solutions, which would decrease the treatment load, including transportation and time consumption.
When evaluating the fidelity of delivery, we considered any session with one or more amendments on the list of actions planned for the session as a delivery failure. If only the number of single actions that failed within a session (and not the entire sessions) was calculated, the progression criteria would probably be on the green level. However, the evaluation of fidelity of delivery on the amber level seems congruent with the need for more education in health behaviour coaching among the OTs, which would provide more confidence in study procedures. Intervention providers’ competence and behaviour are essential determinants of an appropriate intervention delivery.55–57 In general, since occupational therapy is still poorly represented in public multidisciplinary chronic pain treatment in Denmark,58 more opportunities for continued education in the biopsychosocial approach to chronic pain treatment, for example, based on the International Association for Studies of Pain curriculum for OTs,59 would encourage the Danish OTs with interest in the field to get involved. However, we believe that the rigorous professional experience of the intervention OTs was essential. The programme delivery demanded a high degree of coordination, flexibility and reflexivity to meet the participants’ needs, making it a complex task.60 We propose continuous monitoring of the fidelity of delivery at the later research steps to clarify its impact on the outcomes.43
Feasibility studies do not allow for measuring long-term treatment effects.44 The lack of control group and high dropout rates in this study precluded conclusions on the intervention’s impact on HRQoL and occupational performance and satisfaction. However, the improved COPM scores in the entire study cohort supported the previously demonstrated beneficial impact of occupational therapy in chronic pain treatment.61–63 High rates of self-perceived relevance among participants confirmed the necessity of targeting lifestyle and self-determined meaningful activities in chronic pain treatment.64–66 However, the benefits for the everyday life observed seemed not to influence the self-reported HRQoL. Because many participants did not reach MCID for the COPM, and knowing that health behaviour changes are time consuming, further investigation of the short-term and long-term outcomes is needed.
Limitations
This study was bound to specific clinical settings within the Danish tertiary chronic pain rehabilitation. Considering the high heterogeneity in chronic pain treatments identified by other evidence,67 this study’s pragmatic character may have limited its external validity.
Internal, non-blinded assessors and no separate registration files for the baseline and follow-up assessment, for example, in the COPM interview, might have increased the risk of detection bias.68 Qualitative interviews with the participants as a supplemental research activity will shed light on the in-depth opinions of the intervention’s impact.
Using other methods for feasibility evaluation than the red-amber-green method, for example, investigating in clinical utility aspects of the intervention such as possible disturbances it induces in current clinical care, or its social acceptance in different stakeholder groups,69 could help generate new relevant knowledge. Furthermore, this would be relevant because the present feasibility study did not include an assessment of the contextual feasibility, which has been highlighted as important in the 2021 edition of the MRC framework.13 Whether REVEAL(OT) will improve the existing treatment of chronic pain remains unclear. However, including the REVEAL(OT) intervention, we must ensure that the interdisciplinary treatment delivery context considered the golden standard in tertiary chronic pain rehabilitation is secured. One of the essential prerequisites for interdisciplinary cooperation—providing the treatment in the same clinical facilities1—could not become real in the feasibility phase. Therefore, we suggest that the interdisciplinary context of the REVEAL(OT) delivery is secured before launching an RCT.
Conclusion
The predefined research progression criteria for programme adherence, patients’ self-perceived relevance, timing and mode of delivery, assessment procedure acceptance and adverse events demonstrated that the REVEAL(OT) intervention was feasible to deliver. The participants improved their occupational performance and satisfaction. In a future definitive trial, the intervention shall optimise its recruitment, participant retention and the fidelity of delivery strategies by including additional research sites, a revised assessment battery and more flexible delivery solutions.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Regional Committee on Health Research Ethics in Region Zealand (reg SJ-703) and the Data Protection Authority for Region Zealand, Denmark (REG-052-2018). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We want to thank the clinicians from both clinical departments involved in this study for their interest and support and all the outpatients who engaged in it.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @NielsenSolgaard, @STSkou
Contributors As the main author and guarantor for the overall content, SSN was responsible for protocol development, design, planning, conduct, analysis of the results and drafting of the manuscript of this study. STS supervised the protocol development, study conduct, feasibility evaluation methodology applied, analysis of the results and the manuscript drafting. AEL supervised using the occupational therapy assessment techniques (COPM interviews) used in this study and the analysis of the results. JS supervised the study design and conduct and helped draft the manuscript. RP supervised the recruitment procedures, multidisciplinary collaboration and editing of the manuscript. WZP supervised the protocol development and editing of the manuscript. HBV was responsible for managing the digital assessments attached to the PainData registry for this study, supervision of the questionnaire data analysis and editing of the manuscript. JRC participated in the protocol development, design and planning of this study, supervision of the intervention providers and drafting of the manuscript. All authors read, revised and approved the final manuscript.
Funding Grants from Region Zealand Health Research Fund (reg SJ-703), the University of Southern Denmark (reg SSN, the applicant) and the Danish Occupational Therapy Association (reg FF1-18-R76-A1690) financed the first author’s PhD study. STS is currently funded by a programme grant from Region Zealand (Exercise First) and two grants from the European Union’s Horizon 2020 research and innovation programme (MOBILIZE, grant agreement number 801790 from the European Research Council, and ESCAPE, grant agreement number: 945377).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.