Article Text
Abstract
Introduction The pathogenesis of atopic diseases is highly complex, and the exact mechanisms leading to atopic dermatitis (AD) onset in infants remain mostly enigmatic. In addition to an interdependent network of components of skin development in young age and skin barrier dysfunction underlying AD development that is only partially understood, a complex interplay between environmental factors and lifestyle habits with skin barrier and immune dysregulation is suspected to contribute to AD onset. This study aims to comprehensively evaluate individual microbiome and immune responses in the context of environmental determinants related the risk of developing AD in the first 4 years of a child’s life.
Methods and analyses The ‘Munich Atopic Prediction Study’ is a comprehensive clinical and biological investigation of a prospective birth cohort from Munich, Germany. Information on pregnancy, child development, environmental factors, parental exposures to potential allergens and acute or chronic diseases of children and parents are collected by questionnaires together with a meticulous clinical examination by trained dermatologists focusing on allergies, skin health, and in particular signs of AD at 2 months after birth and then every 6 months. In addition, skin barrier functions are assessed through cutometry, corneometry and transepidermal water loss at every visit. These measurements are completed with allergy diagnostics and extensive microbiome analyses from stool and skin swabs as well as transcriptome analyses using skin microbiopsies.
The aim is to assess the relevance of different known and yet unknown risk factors of AD onset and exacerbations in infants and to identify possible accessible and robust biomarkers.
Ethics and dissemination The study is approved by the Ethical Committee of the Medical Faculty of the Technical University of Munich (reference 334/16S). All relevant study results will be presented at national and international conferences and in peer-reviewed journals.
- Eczema
- Paediatric dermatology
- IMMUNOLOGY
- Allergy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Munich Atopic Prediction Study is the first study which collects data at multiple time points from pregnancy through birth and early childhood in the context of atopic dermatitis development in infants.
Phenotypical information includes data from detailed questionnaires, skin swabs, stool and blood sampling, microbiopsies and prick testing.
Intensive follow-up will help to closely track time-to-event data analysis.
Potential limitations include a high dropout rate due to the long follow-up phase.
Introduction
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease characterised by dry, itchy and reddish skin.1 In the last decades, the prevalence of AD in children has increased up to 30% especially in developed countries like Scandinavia, Northern and Western Europe, Australasia and urban areas in Africa, while in Eastern Europe, the Middle East, China and Central Asia a lower prevalence was reported.2 Reasons for the geographic variability are still unclear.2 AD belongs to the most common skin diseases in the northern hemisphere,3 with 60%–80% of affected children experiencing disease onset during their first 6 months of life.4 While AD may resolve in many children later in life, early onset in the first 2 years of life can multiply the risk of a severe and long-lasting disease course including an almost four times higher risk to develop food allergies and 2.5 higher risk to suffer from asthma.5 6 Especially in the severely affected, quality of life of individuals and their families is markedly reduced and the socioeconomic impact of AD is substantial.7–10
Genetic and environmental risk factors are believed to be the main drivers of skin barrier dysfunction and immune dysregulation in the development of AD.11–14 Loss of function in the filaggrin gene is the strongest genetic risk factor for AD pathogenesis and, among others, leads to changes in skin hydration and water retention.15 Genetic modifications seem to be a strong parameter in the development of AD, as particularly shown in familial clusters of the disease.16 Children with a family history of AD present a higher risk of developing AD: if one parent is affected, the risk is increased by around 37%, and if both parents are affected, the risk is increased by nearly 50%.16 However, many children with affected parents remain skin-healthy despite having the same genetic skin barrier defect as their parents. Accordingly, this suggests that other individual influencing factors might be at play such as the living conditions, microbiome–host-interactions, exposure to antibiotics, hygiene standards and daily skin care routine.17 As the pathogenesis of AD is multifactorial and thus the interactions between the factors are highly complex, individual AD aetiology remains unclear.
For better patient and disease management, more in-depth research that examines the relationship between these multiple risk factors is required. In contrast to larger epidemiological studies that focus on dominant population-based risk factors, the Munich Atopic Prediction Study (MAPS) aims to collect highly detailed information and data using a comprehensive study set-up to also examine possible correlations of risk factors for AD in this prospective birth cohort. Using this study design, MAPS aims to characterise the underlying network that drives AD development including the immune and microbial deviations and how environmental factors interact with those markers of alterations and the appearance of AD in the first years of life.
Methods and analysis
Study population and setting
The MAPS cohort is an ongoing prospective and observational birth cohort study conducted on infants from three major hospitals in Munich, Germany. Mothers were actively approached in cooperation with maternity clinics, midwives and dermatologists. In the study, which started in June 2017, mothers who were 18 years and older, were able to fill in a German questionnaire, and stay in the greater Munich area were eligible for participation. Childrens’ parents provided written informed consent prior to study inclusion. There were no exclusion criteria for the newborns. Examinations of the children are scheduled during the first 4 years of life. The present study is an epidemiological project with the establishment of a birth cohort in the Munich area with 300 children. Enrolment of the infants was performed from May 2017 to March 2020. The data collection is completed at the 48th month of life of the participants and will end in March 2024.
Cohort design
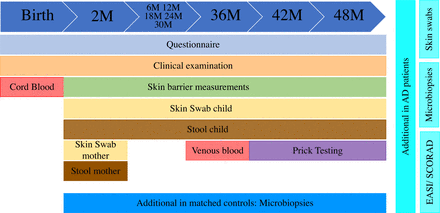
Study procedures are displayed in detail in figure 1 and figure 2. The clinical examination of the children is scheduled at the age of 2 months, at 6 months and every 6 months thereafter at the Department of Dermatology and Allergy of the Technical University of Munich in Munich, Germany (figure 1). All children who develop AD are examined at additional visits as indicated clinically. All examinations are performed by medical doctors trained in dermatology.
Timeline of the scheduled examinations and assessments of children participating in the MAPS cohort over a 4-year timespan. AD, atopic dermatitis; EASI, Eczema Area and Severity Index; MAPS, Munich Atopic Prediction Study; SCORAD, SCORing Atopic Dermatitis.
Overview of samples and materials, their sources and planned analyses for the MAPS data collection. MAPS, Munich Atopic Prediction Study; PBMC, peripheral blood mononuclear cell.
Considering the prevalence of AD in Germany, 15.6% in all children (age<18) up to 22.8% in 1 year old children, it is estimated that of all 300 MAPS participants, approximately 50 infants will develop AD during their first 4 years of life;18 50 affected children with AD would then be compared with 250 healthy children of the cohort and in addition, numerous variables such as for example gut microbiome and skin microbiome as well as physiologic skin measurements would be comparable intraindividually over time in the first years. For the assessment of differences between infants with AD and healthy infants in analyses that require costly laboratory assays, a control group of age-matched and sex-matched healthy children with comparable living conditions (urban, suburban or rural) will be established. Two healthy infants will be included into this control group for every newly diagnosed AD infant.
Parental questionnaires
Mothers are asked to complete questionnaires with questions in regard to their pregnancy, the different developmental stages of their children and circumstantial information. The first questionnaire focuses on the course of pregnancy and associated events. We collected information on drug usage (eg, antibiotics), possible complications during pregnancy (eg, infections), nutrition, living conditions and environmental factors (eg, siblings, pets and the use of disinfectants), sexual health history and hygiene habits (eg, body care). The second questionnaire, filled in when the children reach the age of 2 months, covers the anthropometric measurements (eg, weight, height and body circumference), skin conditions (eg, sunburns), general health issues (eg, wheezing and influenza) of the infant, including any medications, vaccinations, nutrition and environmental factors (eg, smoking of the parents, the presence of siblings and place of residence) and skincare habits (eg, products used and frequency of bathing and moisturising). Starting at the age of 6 months, the third questionnaire is filled in, which focuses on the family and surroundings, mental behaviour of the child-rearing parent(s) (eg, the mother), travels and the child’s health especially recent diseases and vaccinations. At each of the following visits, the questionnaires are asked again (figure 1).
Clinical skin assessment and interviews
At each appointment, patient medical history is taken to obtain information about the child’s health and a clinical examination of the skin is conducted. Parents are asked about recent skin diseases and allergies, externally implemented therapies and diagnostics. Skin inspections are especially brought into focus, which includes assessing skin type (Fitzpatrick classification of skin phototype), the number and localisations of nevi, status of hair, hair pattern, nails and benign skin changes (eg, freckles and accessory mammillae) (figure 1). The skin is evaluated for signs of AD using the diagnostic criteria of Hanifin and Rajka (except for IgE levels).19 The severity of AD is assessed using the Eczema Area and Severity Index (EASI) and the SCORing Atopic Dermatitis (SCORAD).20 21
Non-invasive skin barrier measurements
Skin barrier dysfunction is believed to be one main driver for the development of AD.22 Skin water loss, skin elasticity and skin hydration are all known to be possibly dysregulated in AD and assessed in MAPS23 (figure 1).
The stability and valid outcome of these analyses is highly dependent on a stable setup because the measurements are susceptible to alterations based on the given surroundings. To create stable conditions all year around, these analyses were performed in the same room with air conditioning. Measurements of humidity and indoor temperature were always documented.
Corneometry (stratum corneum hydration)
Corneometry assesses the amount of water in the stratum corneum. In MAPS, the same area is sampled three times in a row using Derma Unit SSC 3 (Courage+Khazaka Electronic, Cologne). The skin provides a diaelectric medium and the water content correlates with the electric capacity of the skin. Measurements are always performed on the cubital fossa by applying light pressure.
Cutometry
The elasticity of the skin is assessed by cutometry using the Cutometer Dual MPA 580 (Courage+Khazaka Electronic, Cologne). Negative pressure, created in the device, deforms the skin mechanically. The skin is drawn into the aperture of the probe and released again after a defined time while exposed to a non-contact optical measuring system. Conclusions can be drawn about the elasticity and firmness. Measurements are repeated three times on the same area of the arm.
Transepidermal water loss (TEWL)
TEWL is measured using the portable, closed condenser-chamber device AquaFlux model AF200 (Biox Systems, UK). TEWL is used to characterise the membrane function of the epidermis by calculating the capacity of diffusion of water. Measurements are repeated three times on the cubital fossa.
Skin and nose swabs
At each study visit, skin swabs of the crook of the arm and the nose are collected using DNA-free nylon flocked swabs (figure 1). Swabs are moistened with 0.15 mM NaCl solution containing Tween 20 at 0.1%. Skin microbiome samples are obtained by rubbing back and forth the swab approximately 50 times and applying firm pressure. Nose microbiome samples are collected using swabs soaked in NaCl solution without Tween 20. The children were not supposed to be bathed or creamed 24 hours before a visit. At the 2 months visit, the mother’s skin is sampled as well. Swabs are taken from the volar forearm of all mothers. The standards are the same as used in the children. In all children who develop AD, skin swabs are collected from affected and non-affected areas of the skin. DNA from living microbes is extracted.24 Individual bacterial colonisation is analysed using 16S-rRNA gene sequencing. Key samples will be analysed by metagenomics sequencing to investigate the metabolic capabilities of the microbiome and to identify microbes at a higher resolution (figure 2).
Microbiopsies
Because of the invasive nature of the conventional biopsies, we perform a minimally invasive approach using microbiopsies where neither local anaesthesia nor suturing are necessary. These devices are designed to collect skin samples of 0.21 mm in diameter and of 0.26 mm in depth.25 26 Microbiopsies are performed when AD is observed in a child during a study visit or an additional appointment made (figure 1). Samples are collected from lesional and non-lesional areas (perilesional at a distance of 5–10 cm) in addition to corresponding sites from healthy matched controls. Microbiopsy skin samples are stored in RNA stabiliser at −80°C until whole RNA preparation. Skin transcriptomes will be analysed by sequencing cDNA libraries created from the mRNA in these preparations (figure 2).
Stool sampling
Stool samples are taken from mothers at the 2 months visit and from children at each appointment using Stool Collection Tubes with DNA Stabiliser (Invitek Molecular, Berlin, Germany) (figure 1). DNA is extracted from stool samples using the ZymoBiomics DNA miniprep kit (Zymo Research, Freiburg, Germany) and analysed for the 16S-rRNA gene as described for skin and nose samples. Furthermore, metabolome analyses from key samples will be performed to investigate short chain fatty acid levels and other immune modulators (figure 2).
Cord blood
Immediately after birth, 80 mL of blood is obtained from the umbilical cord veins of the newborns and collected in a Vita 34 collection bag (Vita 34 AG, Leipzig, Germany) (figure 1). The blood is further processed for three different investigative approaches: (1) identification of different cell populations by flow cytometry, (2) plasma collection for determination of systemic cytokine and antibody levels and (3) peripheral blood mononuclear cell (PBMC) isolation for in depth characterisation of specific cell types (eg, stimulation experiments, expression and epigenetic analysis). To determine different cell populations by flow cytometry, a multicolour staining in whole blood was established (figure 2).
Blood sampling
At the age of 36 months, blood is collected from all children still participating in the study to obtain serum, EDTA-blood and citrated blood (figure 1). A multiplex assay (ImmunoCAP ISAC, ThermoFisher, Waltham, USA) will be used to determine specific IgE against allergen components in serum, while differential blood counts for neutrophils, eosinophils, basophils, monocytes and lymphocytes are obtained from EDTA blood. In children diagnosed with AD and appropriate controls (sex-matched, age-matched, birthmonth, place of residence-matched), flow cytometric analysis and isolation of PBMCs are additionally performed from citrate blood analogous to cord blood (figure 2).
Prick test
Skin prick test is a commonly used functional test to detect sensitisation. Different substances are applied to the surface of the skin and a small prick through the drop is made to the skin using a sterile prick lancet. In the fourth year, a prick test is performed on all study participants using standard aero-allergen and childhood food allergens under standard conditions (figure 1). The later include cow’s milk, hen’s egg, wheat, soybean, Dermatophagoides pteronyssinus, grass pollen, hazelnut and cod. Skin reactions are determined 15 min after the scratch.
Research objectives
To establish:
Overall proportion and characteristics of infants who develop AD, including early childhood development, anthropometric measurements (eg, weight, height, body circumference), skin conditions (eg, sunburns), general health issues (eg, wheezing, influenza), medications, vaccinations, nutrition and environmental factors (eg, smoking of the parents, the presence of siblings and place of residence) and skincare habits (eg, products used and frequency of bathing and moisturising), mental behaviour of the child-rearing parent(s) (eg, the mother), travels and the child’s health especially recent diseases and vaccinations.
The role of the mother in AD development, including characteristics of the mother, behaviour in pregnancy (information on drug usage (eg, antibiotics), possible complications during pregnancy (eg, infections), nutrition, living conditions and environmental factors (eg, siblings, pets, the use of disinfectants), sexual health history and hygiene habits), possible early AD biomarkers in cord blood
The role of the microbiome in AD development, including skin-barrier function.
Biomarkers in the cord blood to predict AD development
Data management
The collected data are entered directly into an online REDCap (Research Electronic Data Capture). REDCap is a metadata-driven browser-based workflow methodology, supporting clinical and translational research by rapid development and deployment of electronic data.27
Patient and public involvement
Neither patients nor the public were involved in the development of the research question or the study design. The study was planned by an interdisciplinary research team consisting dermatologists, epidemiologists, gynaecologists, biologists and biochemists. The results will be disseminated to study participants via open-access publications.
Statistical analysis
Descriptive statistic will be used for all dimensions collected at baseline and the follow-up questionnaires separately. Furthermore, we will conduct adjusted regression analyses to understand associations between exposure and AD at different time points. The following independent variables regarding AD will be examined: sex (female, male), birth season (spring-summer, autumn-winter), delivery via C-section, siblings, total duration of breastfeeding, intake of antibiotics, analgetics, cold remedy, vaccination (all of them at different time points: pregnancy and childhood), maternal education, maternal employment before maternity leave, maternal AD, paternal AD, sibling with AD, maternal depression, maternal anxiety, maternal smoking, maternal chemical hair treatment, maternal cold during pregnancy, maternal acute febrile disease during pregnancy, residence (urban and rural), living close to traffic road and pet owner. To identify associated factors of developing AD, univariate logistic regression models will be conducted and OR will be calculated. All aforementioned independent variables will be included in the base model. Based on variable selection, a final multivariate logistic regression model will be calculated to construct a risk prediction model for AD. Analysis of multicollinearity among the predictors will be performed by calculating the phi coefficient. Cord blood analyses will be carried out using the FlowJo data anlysis software package (TreeStar, USA). Mann-Whitney-U test will be used to compare whether there are differences in the dependent variable for two independent groups (AD vs healthy).
For microbiome data analyses, the generated 16S amplicons will be processed following the UPARSE method and the obtained clean reads are used for diversity analysis, taxonomy binning, serial group comparison and correlations.28 The generalised UniFrac will be used for calculation of the phylogenetic distance matrix and the Bray Curtis method to assess similarity between samples. The non-parametric Kruskal Wallis Rank Sum test and Mann-Whitney-U test, respectively, will be used for multiple and pairwise group comparisons. Multiple test corrections will be performed with the Benjamini and Hochberg procedure. For transcriptome data analysis, reads will be normalised to counts per million (CPM) and only genes with levels above 0.5 CPM will be retained. The DESeq2 package will be used to identify the differentially expressed genes (fold change≥1.5, adj.p<0.05 and false discovery rate <0.05).29 Pathway analysis will be performed using the gene ontology analysis approach including a comparison of different data bases as biological process, KEGG (Kyoto Encyclopaedia of genes and genomes), Biocarta and reactome. Gene set enrichment analysis will be carried out using the Gene Set Enrichment Analysis (GSEA) platform.
Strengths and limitations
MAPS is the first German birth cohort investigating the role of possible risk factors in the development of AD in a prospective manner. Detailed studies of birth cohorts can assess many factors simultaneously.30 Data from children, including their development, are regularly assessed in questionnaires, clinical examinations, examinations of the skin barrier and skin, stool microbiome samples, skin transcriptome analysis and genetic and epigenetic blood analysis.
MAPS aims to understand AD using a multifactorial and holistic approach to identify complex and previously unknown mechanisms.
Potential limitations include a high dropout rate due to the long follow-up phase. Significant parameters in families’ life could be changing living conditions due to having a baby or moving to another area. Another limitation is that not all socioeconomic strata and thereby living conditions are equally represented. Selection bias is another limitation, since parents who themselves have AD might be more willing to participate than non-affected parents. Multiple imputation models may be needed if the number of cases is small.
Strategic aims for the upcoming years
The birth cohort is set up for 4 years. The focus for the upcoming years is to keep participants engaged in the study through regular follow-up visits every 6 months to minimise potential dropouts. With the large volume of data collected over the 4-year timespan, extensive analyses including several topics related to AD development in infants can be carried out. Combined with the already existing studies on individual risk factors on AD, we hope to expand on the evidence currently available in the literature and to identify areas which are hardly studied. To visualise, analyse and structure the data, network analysis will be used to identify potential clusters within the large data sets.31 Overall, the study aims to better understand the underlying mechanisms of AD and the interaction of various risk factors in infants to improve patient care and disease management.
Ethics and dissemination
The study is approved by the Ethical Committee of the Medical Faculty of the Technical University of Munich (reference 334/16S). Childrens’ parents provided written informed consent prior to study inclusion. All relevant study results will be presented at national and international conferences and in peer-reviewed journals.
Ethics statements
Patient consent for publication
References
Footnotes
SP and LS contributed equally.
Contributors TB and AZ designed and directed the project; SP, VL, RB, AD, SS, AB and CS performed the measurements; LS, LT, MS, LM, HH, BK, EH, MS, SN, DA, SE, RLS, MH, BE, SK, MK, YA, YS and ZK were involved in planning and supervised the work. SP wrote the manuscript in consultation with LS.
Funding Helmholtz Zentrum München, German Research Center für Enviromental Health GmbH, Clinical Unit Allergology (EKA); Deutsche Forschungsgemeinschaft (DFG), GRK2668.
Competing interests TB gave advice to or received an honorarium for talks or research grants from the following companies: ALK-Abelló, Janssen, Meda, Novartis, Phadia Thermo Fisher, Sanofi and Celgene. AZ gave advice to or received an honorarium for talks or research grants from the following companies: ALK-Abelló, Janssen, Novartis, Phadia Thermo Fisher, Sanofi and Leo Pharma. BK gave advise and received an honorarium for talks from ITF-company. LT received an honorarium for talks on research grants from the following companies: Janssen, Novartis and Beiersdorf Dermo Medical.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.