Article Text
Abstract
Objectives This systematic review aims to compare the effects of active monitoring and abduction treatment on the Graf alpha angle, Acetabular Index (AI) and femoral head coverage in infants with stable developmental dysplasia of the hip (DDH).
Design Systematic review reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data sources A search of the PubMed, Embase, Cochrane and Web of Science databases was performed in January 2020 and updated in January 2021.
Eligibility criteria (Non-)randomised studies comparing active monitoring with abduction treatment in infants younger than 4 months with stable DDH were included.
Data extraction and synthesis All eligible articles were methodologically assessed using the Cochrane risk of bias tools. Data were extracted by summarising the study characteristics and results.
Results Of the six included studies, two randomised studies were of low risk and two of some concerns. Two non-randomised studies were of serious risk. In total, 544 dysplastic hips (439 infants) were investigated, of which 307 were observed and 237 were treated. Two studies reported a faster improvement of the alpha angle and average acetabular coverage in treated hips at 3 months. No differences in AI between the treatment and observation group after 3 months were reported. In total, 38 infants (12%) in the observation group switched to the treatment group. At the final radiograph, 21 observed hips and 32 treated hips were dysplastic.
Conclusions There were no differences in AI between the treatment and observation group after 3 months in infants up to 4 months of age with stable DDH hips. The switch of 38 infants (12%) from the observation to the treatment group corroborates that not all infantile DDH hips will spontaneously progress into normal hips. The small study population sizes and methodological heterogeneity warrant a large randomised controlled trial to study this research question.
PROSPERO registration number CRD4202123300.
- paediatric orthopaedics
- hip
- diagnostic radiology
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
All identified studies, independent of the quality of the studies, were included in this systematic review. Thus, providing a complete overview of current literature.
Risk of bias of the included studies was extensively reviewd.
Great heterogeneity in measurement methods and measurement moments of the included studies, made it difficult to compare study results and impossible to perform a meta-analysis.
There was great heterogeneity in the quality of the included studies, since two non-randomised studies classified as serious risk of bias.
Introduction
Developmental dysplasia of the hip (DDH) is one of the most common paediatric orthopaedic disorders in newborns and young children.1 2 DDH comprises a spectrum of developmental hip abnormalities ranging from mild dysplasia of the acetabulum to dislocation of the femoral head.3 4 The incidence rate of DDH differs per geographic location, ethnic background and diagnostic definition and varies between 1/1000 and 20/1000.2 4 5 Untreated DDH can result in short-term and long-term morbidity, such as, chronic pain, gait abnormalities and early hip osteoarthritis.4 6 To detect DDH at an early age, screening programmes have been implemented worldwide.
Controversy exists on the optimal screening method to detect DDH (universal screening vs selective screening) and timing differs considerably worldwide.7 8 In the Netherlands, all newborns are screened for DDH within the first month after birth by the Dutch national screening programme. When newborns present with an abnormal clinical examination (knee height, passive hip abduction and the Ortolani and Barlow manoeuvres) or when risk factors (family history, breech position) are present, the newborn is referred for an ultrasound at the age of 3 months. If there is a suspicion of luxation, the infant is referred for an ultrasound within 2 weeks.8 9 In Europe, selective screening is also used in Belgium, France, Portugal, Sweden, Norway, Hungary, the UK and Ireland. Conversely, Austria, Germany, Switzerland, Italy, Slovenia and Slovakia use a universal ultrasound screening method.7 The timing of ultrasound screening ranges from week 1 to week 12.7 A third screening method is universal screening including clinical examination only.10 Existing literature comparing screening methods is scant and shows methodological heterogeneity.7
Limitations of clinical examination alone are the lower sensitivity, difficulty to identify subtle signs and the majority of positive Ortolani or Barlow manoeuvres will spontaneously resolve within 2–4 weeks after birth.10 11 Ultrasonography according to the Graf method is one of the most used methods to diagnose and classify DDH.12 13 The Graf method classifies type two hips as stable but dysplastic hips and type three hips as unstable or luxated hips.9 Hip ultrasonography facilitates the ability to identify smaller anomalies, thereby possibly introducing overdiagnosis.11 A study by Roovers et al suggests that 85% of infantile DDH will resolve by the age of 3 months without treatment initiation.14 The hypothesis that stable hips tend to spontaneously progress into normal hips is supported by current literature.6 15 Currently, abduction treatment is the most opted DDH treatment in children younger than 6 months.16 However, it is debatable whether abduction treatment alters the natural course of stable hips.6 A study by Pollet et al did not find a difference in acetabular development between abduction treatment and active monitoring in infants with stable hips at the age of 3 to 4 months.2 Therefore, the preeminent question is whether stable hips (Graf type 2) are truly pathological and warrant abduction treatment.6 Furthermore, abduction treatment might expose the infant to complications, such as avascular necrosis (AVN) of the femoral head and transient femoral nerve palsy.3 A systematic review of the literature is needed to summarise existing studies comparing abduction treatment and active monitoring in stable hips. The results of this systematic review might impact current screening and treatment methods and will identify knowledge gaps.
The aim of this systematic review is to compare the effects of active monitoring and abduction treatment on the Graf alpha angle, Acetabular Index (AI) and femoral head coverage (FHC) in infants with stable DDH (Graf type 2).
Materials and methods
Search strategy and protocol
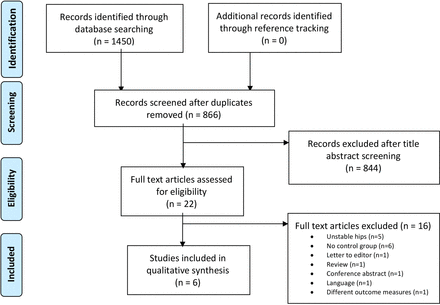
This systematic literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines17 (online supplemental appendix 1). A flowchart of this process is depicted in figure 1. The databases PubMed, Embase, Cochrane and Web of Science were systematically searched in January 2020. The search was updated in January 2021. Citation software (Endnote V.X9.3.3, Clarivate Analytics, Boston, Massachusetts) facilitated the search strategy. A Boolean for the search string with the used keywords and index terms (Mesh headings) is provided (online supplemental appendix 2).
Supplemental material
Flowchart of the selection process with reasons for exclusion based on full text.
Study selection
The search string was developed in consultation with a research librarian. After eliminating duplicates, the identified articles were screened by NMCM and MAW based on title and abstract. Interreviewer disagreements were solved by consensus and with assistance EMBP. Articles considered relevant by title and abstract were read in full text by EMBP, NMCM and MAW to determine final eligibility. To complete the search, reference lists of relevant articles were screened and Google Scholar was used for forward citations by EMBP.
Eligibility criteria
Studies investigating infants younger than 4 months of age presenting with stable DDH were included in this review. Studies were eligible for inclusion when presenting at least one of the following outcome values: Graf alpha angle, AI or FHC. Studies including participants with major congenital abnormalities, such as cerebral palsy or spina bifida, were excluded.
The search was restricted to the English and Dutch language. Randomised controlled trials (RCTs), pseudo-RCTs and non-randomised studies were included. For non-randomised studies, both prospective and retrospective studies with two groups (including case–control studies) were included. Studies without comparator (ie, not comparing active monitoring with abduction treatment), cross-sectional studies, case series and case reports were excluded to ensure the inclusion of high level of evidence studies.
Risk of bias
The quality of the studies was assessed by three reviewers using the revised Cochrane risk of bias tool for randomised trials (RoB 2.0) and the Cochrane tool for risk of bias in non-randomised studies (ROBINS-I). All items—that is, selection, performance, attrition, detection and reporting bias for randomised studies, complemented with confounding and recall bias for cohort studies—were rated accordingly. Since blinding of caregivers and patients was not possible due to the nature of the intervention, this aspect of performance bias was assessed less strictly for all studies. The overall risk of bias was attributed as low risk, some concerns or high risk for the randomised and low, moderate, serious or critical risk for the non-randomised studies (online supplementary appendices 3 and 4).18 19
Outcomes and data abstraction
To compare the included studies, one author extracted the following characteristics: inclusion and exclusion criteria (degree of dysplasia, age at time of inclusion, comorbidities, previous treatment), subject characteristics (gender, treatment allocation), used abduction device, follow-up moments, outcome measures (Graf alpha angle, AI and FHC), changes in treatment allocation and study conclusions. This process was reviewed by a second author. Effect sizes were calculated for each study based on means, SD and number of infants/hips using an online calculator.20
Patient and public involvement
Due to the nature of this study, patients were not involved in the development of the research question, design and conduct of this study. The outcomes of this systematic review will be reported to the Dutch patient association for developmental hip anomalies ‘Vereniging Afwijkende Heupontwikkeling’.
Results
Study identification
The initial search provided 1450 records of which 866 remained after removal of duplicates. No additional articles were obtained through reference tracking. All 866 articles were screened by title and abstract. Among these, 22 articles remained eligible for full text review, of which 6 were selected for quality assessment and data extraction. The reasons for exclusion by full text are outlined in the PRISMA flowchart (figure 1).
Selected articles
The six included studies consisted of four RCTs (Wood et al,21 Rosendahl et al,22 Brurås et al23 and Pollet et al2) and two non-randomised studies, of which one was a retrospective (Sucato et al24) and one was a prospective cohort study (Kim et al25). One RCT (Brurås et al23) was a long-term follow-up of another eligible study (Rosendahl et al22).
Risk of bias assessment
Two of the four RCTs were rated as low risk of bias (Rosendahl et al22 and Brurås et al23) and two of some concerns (Pollet et al2 and Wood et al21). The two non-randomised studies24 25 were rated as serious risk of bias (online supplemental appendices 3 and 4).
Cohort description
A total of 544 hips were investigated in the included studies. Of these, 307 were actively observed with ultrasound and radiograph and 237 were treated with an abduction device. These numbers do not comprise the 83 hips of Brurås et al since they were also included in the study of Rosendahl et al.22 23 Of the 544 hips, at least 97 hips were Graf type IIb and 152 type IIc. However, not all studies reported Graf types (Wood et al,21 Kim et al25) and one study included stable hips with other Graf types than IIb and IIc (Sucato et al24).
The total 544 hips belonged to 439 infants. Of these 439 infants, 357 were female and 82 were male (table 1).
Overview of study characteristics of the included studies and the total number of included hips and infants
Treatment strategies
All randomised studies assigned their patients to either observation (active monitoring), with ultrasound and radiograph evaluation, or abduction treatment, with Pavlik Harness or Frejka Pillow, at the time of inclusion. In the non-randomised studies, treatment was decided based on the discretion of the treating physician (table 1).
The age of the infant at inclusion varied from 1 day to 4 months. Follow-up was performed with ultrasound and radiograph and the maximum follow-up duration ranged from 3 months to 6 years (table 1). If sufficient progression of hip development was found, treatment was discontinued in the treated infants. Sufficient progression was defined as: the acetabular coverage to have become normal (greater than 50% cover) at 6 weeks or if the radiograph was normal (showing no signs of dysplasia and an acetabular angle of <30°) at 3 and 4 months21; an alpha angle>53° at 6 weeks or an alpha angle≥55° at 3 months or an AI of ≤2 SDs above the mean at 6 months22 23; improvement of the alpha angle at 6 or 12 weeks2; an alpha angle≥60°/Graf type 1/non-convex shape of the acetabulum/coverage of the femoral head of ≥50% in the non-stress view or ≥40% in the stress view or an AI of ≤2 SDs above the mean24; or an alpha angle≥60° and FHC≥50% or an AI≤2 SDs above the mean.25 In case of insufficient progression or deterioration of the dysplasia, treatment was initiated in the observed infants or continued in the treated infants (table 2). The number of infants in the observation group that switched to the treatment group are reported in the ‘Treatment switch’ column in table 2.
Overview of the results and conclusions of the included studies
Radiological results
Two studies reported statistically significant differences in alpha angle or average acetabular coverage between observed and treated infants at 3 months.21 22 One of these two studies also showed an increased treatment effect of abduction treatment compared with observation at 1.5 and 3 months.22 After 3 months, none of the studies showed statistically significant differences in AI between the treatment group and observation group. Also, one study did not show an increased treatment effect of abduction treatment compared with observation at 12 months.22
Three of the six included studies reported that infants in the observation group had switched to the abduction treatment group. Reasons for this switch were an alpha angle<50° at 6 (n=11) or 10 weeks (n=1), an alpha angle<55° at 3 months (n=12) or an AI>2 SDs above the mean (n=5),22 deterioration of the alpha angle at six (n=3) or 12 weeks (n=7)2 and persistent ultrasonic dysplasia (n=2).25 In total, 38 infants (12%) in the observation group switched to the abduction group.
At the end of the follow-up duration, 21 observed hips and 32 treated hips were still dysplastic. One study, examining the long-term effects of abduction treatment and observation in the study population of Rosendahl et al, reported zero observed and one treated hip to still be dysplastic at the age of 6 years (table 2). From the treatment group, two infants received an arthrogram without further surgical intervention,21 one infant had a Salter osteotomy,23 and two infants were treated with closed reduction and spica cast.2 None of the infants of the observation group had a surgical intervention.
Discussion
This systematic review explores one of the most pressing questions in DDH care, namely whether abduction treatment alters the natural course of stable DDH hips. This systematic review suggests that there are no differences in outcome between abduction treatment and observation in infants up to 4 months of age with stable DDH hips. Two studies reported a faster improvement of the alpha angle and average acetabular coverage in stable DDH hips that received abduction treatment at 3 months.21 22 However, none of the six studies reported differences in AI between the treatment and observation group after 3 months.
A total of 38 infants (12%) in the observation group switched to the abduction group. This finding supports current literature that 80%–85% of stable DDH hips will spontaneously progress into normal hips.6 14 Thereby adding evidence to the hypothesis that ultrasonography is not able to differentiate between truly pathological hips and immature hips.6 In all studies, treatment switch was based on radiological characteristics. Although exact radiological definitions differed between studies, complicating the comparison of results. Also, two of the three studies in which infants switched groups reported that results were analysed according to the intention-to-treat principle. This might result in more optimistic results of the observation group. However, the intention of active monitoring is to actively monitor and intervene when necessary. Therefore, the intention-to-treat principle might be the best approach to represent the clinical situation. The switch of infants from the observation group to the treatment group corroborates that not all infantile DDH hips will spontaneously progress into normal hips. Possible disadvantages of active monitoring are that if treatment is warranted at a certain point, treatment is initiated at a later age and the treatment duration might be longer. However, one study reported that the median treatment duration was similar in the observation group and treatment group, namely 12 weeks.22
One of the included studies found no correlation between the severity of Graf classification at birth and the subsequent presence of DDH.24 None of the other studies examined predictors of final radiographic outcome. It might be argued that early screening results in the diagnosis of more infants with hips that will spontaneously progress into normal hips and that later diagnosis will include more truly pathological hips. This hypothesis is supported by a recent prospective cohort study.26 This study proposes screening at the age of 2 or 3 months or implementation of a wait and see policy for immature hips. Active monitoring around 2 or 3 months of age might aid in detecting late and truly pathological DDH hips while limiting overtreatment, as supported by this systematic review. However, none of the included studies analysed the relationship between initial age at diagnosis and final radiological outcome.
Limitations
The principal limitation of this systematic review is the methodological heterogeneity between the included studies. Age at diagnosis, (radiological) criteria for diagnosis and classification, follow-up schemes and criteria to initiate treatment in the observation group showed great variety. For instance, although all hips included in this review were classified as stable, only some could be attributed to Graf type IIb or IIc. Also, definitions of sufficient hip progression on ultrasonography varied between the included studies. Currently, normal values and values for truly pathological hips in infant hip ultrasonography are lacking.2 This heterogeneity has limited the comparison of study results and a meta-analysis was not feasible. Also, the study quality varied for the included studies, with two non-randomised studies classified as serious risk of bias. After careful consideration, we have decided to include these two studies in this review to present a complete overview of current literature. Finally, the study of Burås et al is a 6-year follow-up derived from the study of Rosendahl et al and was included to gain insight on long-term outcomes. Since both studies included the same infants, the study of Burås et al was not used for calculating the total number of infants (female, male), hips (observed, treated, Graf type) and treatment switches reported in this review (tables 1 and 2).
Future directions
This systematic review suggests that abduction treatment and observation (±delayed treatment) do not result in different outcomes in infants up to 4 months of age with stable DDH hips. However, the included studies have small population sizes and show considerable methodological heterogeneity. Therefore, a RCT is warranted to study this research question in a large population. Ideally, RCTs would be embedded in current standard care follow-up routines. Since differentiating between truly pathological hips and immature hips that will naturally progress into normal hips is currently impossible, this research question remains the most pressing question in DDH care. Consequently, the development of an ultrasound classification system that will distinguish truly pathological hips from immature hips should be pursued. Also, the relation between patient demographics (e.g., age at diagnosis) and radiological criteria, as well as the relation between final radiological outcome and need to switch from observation to treatment group should be further explored. Prospective cohort studies using national registries might play an important role. Furthermore, the cost-effectiveness of observation compared with abduction treatment should be explored in a large trial.
Conclusion
Whereas two studies reported a faster improvement of the alpha angle and average acetabular coverage in stable DDH hips that received abduction treatment at 3 months, none of the six studies reported differences in AI between the treatment and observation group after 3 months in infants up to 4 months of age with stable DDH hips. The switch of 38 infants (12%) from the observation group to the treatment group corroborates that not all infantile DDH hips will spontaneously progress into normal hips.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank Dr G Franssen for the support with developing the search string.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
EMBP and FECMM contributed equally.
Contributors NMCM and MAW were involved in the study design and developed the search string; EMBP, NMCM and MAW performed the literature search, extracted data and performed the risk of bias analysis. All authors read and approved the final manuscript and were involved in writing the manuscript. EMBP and FECMM contributed equally to this paper. MAW is responsible for the overall content of this article as guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.