Article Text
Abstract
Objectives To determine which factors contribute to the decision of mothers to participate with their child in follow-up (FU) examinations after participation in a randomised controlled trial (RCT) prior to conception.
Design A cross-sectional survey, including Likert-scale items. Comparisons will be made between respondents who participated in all FU rounds of data collection and those who did not participate in any FU round with their child.
Participants Women who participated in an RCT investigating the effect of a preconception lifestyle intervention (LIFEstyle study: Netherlands Trial Register: NTR1530) were invited to participate with their child in three FU data collections when the child had a mean age of 4.2 years, 4.6 years and 6.5 years, respectively. FU rounds included a health questionnaire, physical examination and cardiac assessment, successively.
Results Sixty-seven respondents were included, of whom 7 (10%) did not participate in any FU round and 24 (36%) participated in all FU rounds. Women who participated with their child in all 3 FU data collection rounds felt more involved in the FU research (95.8%) and agreed more often that the FU was introduced well (91.7%) as compared with women that did not participate in any FU data collection round with their child (14.3% and 28.6%, respectively). Participants of FU rounds more often agreed that participation felt like a health check for their child as compared with non-participants. In addition, participants of the physical examination and cardiac assessment more often let their decision to participate depend fully on their child, as compared with non-participants (39.4% vs 17.7% and 52.5% vs 24%, respectively).
Conclusions To increase participation rates in future FU studies of children after maternal participation in an RCT, we suggest to involve women in the design of the FU study, to emphasise possible perceived benefits of participation and to encourage women to actively involve their child in the decision of participation.
- EPIDEMIOLOGY
- PUBLIC HEALTH
- Community child health
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. Please contact authors for information on data availability.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
We designed a questionnaire to determine which factors influence the decision of mothers to participate with their child in follow-up (FU) examinations after participation in a randomised controlled trial prior to conception.
The questionnaire was piloted among randomly picked women to ensure all possible factors were addressed in the questionnaire.
We compared respondents who participated in all three FU rounds of data collection to those who did not participate in any FU round with their child.
All respondents answered the questionnaire at one moment in time and after completion of the FU, thus a change in opinion during FU was not accounted for.
Introduction
Maternal health before and during pregnancy is associated with health outcomes in children throughout the life course.1 2 Observational studies have shown that maternal health conditions before or during pregnancy, such as obesity and diabetes, are associated with an increased incidence of obesity, type 2 diabetes and hypertension in their children.3–5 Interventions before or during pregnancy could potentially affect children’s health in the long run. In order to assess causal effects of such interventions on children’s health, long-term follow-up (FU) of randomised controlled trials (RCTs) evaluating interventions before or during pregnancy is needed.
Currently, only 16% of RCTs evaluating effects of interventions during pregnancy include an FU to evaluate the effect of the intervention on the child’s health.6 This low number may be due to the high costs and long timespan that exceeds most funding schemes, as well as logistical and legal challenges.7 An important challenge which hampers the unique ability of trials to assess causality is that such long-term FU studies in children of mothers who participated in RCTs investigating effects of interventions before or during pregnancy often face high loss-to-FU. Loss-to-FU can induce selection bias, leading to imbalances in study groups, which can jeopardise the ability to assess causality.8 9 Importantly, the validity of the study results correlate directly with the degree of loss-to-FU.10
The importance of the preconception period in determining the long-term health in children has been well established and recognised by several important authorities, including the WHO and the International Federation of Gynaecology and Obstetrics.2 11–19 Studies aimed at improving preconception health in women with obesity are conducted more often and should be seen as a public health priority.11 20–22 With the alarming rise of maternal obesity worldwide, the effect of preventive strategies on the detrimental effects of maternal obesity on long-term health in children is necessary, and high follow-up rates must be ensured.14 23 To minimise loss-to-FU in this type of FU, an understanding of factors that influence the decision for participation is important. For this semi-qualitative study, we included women who participated in an RCT investigating the effects of a lifestyle intervention before pregnancy on fertility outcomes in women with obesity. During the FU, which was introduced after inclusion for the RCT, children born to these women were invited to participate in several FU data collection rounds to investigate their long-term health.24 The FU rounds in the children included a questionnaire addressing the child’s health, a physical examination near their homes and a cardiac assessment in a hospital. We aimed to determine which factors play a role for mothers when deciding whether or not to participate with their child in FU research. Eventually, our results could be implemented in the design of future FU studies of children after maternal participation in an RCT, and eventually limit loss-to-FU.
Methods
Participants
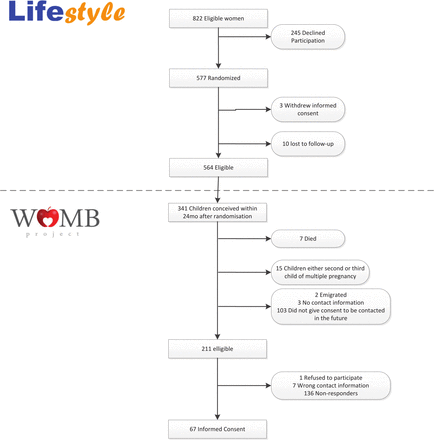
We included women who participated in the LIFEstyle study, an RCT investigating a preconception lifestyle intervention.25 The intervention study included infertile women with obesity and these women were randomly assigned to a lifestyle intervention before fertility care or prompt fertility care.25 Women were eligible if they conceived a healthy child within 24 months after randomisation in the LIFEstyle study, had given permission to be contacted for FU research of their child and had given available contact information.25 The FU study was set up to evaluate the long-term health in both women who participated in the RCT and their children.24 In this study, we focused solely on the FU of the children. The FU in the children consisted of three consecutive rounds of data collection in a period of 8 years after randomisation (see figure 1). Table 1 demonstrates an overview of the mean age and FU rates of the children during the different FU rounds. In summary, during the first FU round, the children had a mean age of 4.2 years and mothers were asked to fill in a health questionnaire addressing the child’s general health and behaviour as well as monitoring the child’s food intake 3 times in 1 week. In addition, an accelerometer was provided to measure the physical activity of the children. The second round, the physical examination, consisted of a one-time visit to a mobile research vehicle near the family’s home when the children had mean age of 4.6 years. We measured anthropometry, body composition, cardiometabolic health and behavioural components.26 During the physical examination, participants were asked to give consent for an additional buccal swab, faeces sample and/or blood sample to gain more insight in the biochemical and genetic profiles. The third FU round was a cardiac assessment in a hospital when the children in the study had a mean age of 6.5 years. This cardiac assessment consisted of an echocardiogram and a cardiac MRI study. Participation during this round took approximately 1 hour for the echocardiogram and an additional 1 hour for the cardiac MRI.
FU data collection rounds. FU, Follow-up.
Overview of the FU data collection rounds
FU participation questionnaire
We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional reporting.27 All eligible participants were asked to complete a questionnaire with statements regarding participation in FU research of their child (see online supplemental figure 1) and provide written consent. The participation questionnaire consisted of two parts. The first part addressed topics including (1) experience during the original intervention study, (2) communication to participants, (3) knowledge and stigma of the subject of research and (4) understanding of the importance of the research topic. The second part consisted of statements specific for the FU round and were asked separately for each FU round to determine which factors played a role in participation for each round. These statements included: (1) I let the decision of participation depend fully on my child, (2) my child was too young to participate, (3) participation would feel like a health check for my child, (4) the distance to the research location would be too far, (5) the research visit would be too burdensome for my child and (6) the research visit would take too much time.
Supplemental material
In total, the questionnaire included 70 statements and mothers had to indicate how much they agreed on a 5-point Likert scale: 1 stated ‘strongly disagree’, 2 stated ‘disagree’, 3 stated ‘neutral’, 4 stated ‘agree’ and 5 stated ‘strongly agree’. Apart from the Likert scale, we used multiple choice and open questions.
Patient and public involvement
Participants were involved in the conduct of this research. During the feasibility stage, we pretested the questionnaire among 10 participants to optimise coverage of questions and assure clarity of the questions. Based on their feedback, we added two questions to the questionnaire: ‘If the follow-up study would have been introduced by someone from the original study team, I would have been more likely to participate’ and ‘The link between the original intervention study and the follow-up study was clear’ (online supplemental figure 1).
Data analysis
For the analysis, we combined 4 (agree) and 5 (strongly agree) to summarise the percentage of agreement. To assess which factors contributed to the decision to participate in the study, we compared the answers of respondents that participated in all three FU rounds with respondents that did not participate in any FU round with their child. In addition, we compared the level of agreement between participants and non-participants within each FU round to determine if there were certain factors associated with participation for a specific type of FU. Comparisons between groups were made using Fisher’s exact test. The analyses were performed using IBM SPSS V.26. A p value of <0.05 was considered statistically significant.
Sensitivity analysis
To assess possible selection bias, we compared our group of participants with all eligible non-participants.
Results
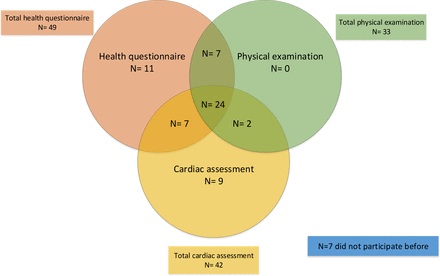
In total, 341 children were conceived within 24 months after randomisation and 211 dyads were eligible and approached (see figure 2). Sixty-seven respondents (31.8%) completed the FU participation questionnaire. For an overview of the respondents and their previous participation in FU with their child, see figure 3. Table 2 demonstrates the baseline characteristics of the respondents who completed the questionnaire. See online supplemental table 2 for the STROBE checklist.
Participation flowchart.
Distribution of respondents and previous FU participation with their child. FU, follow-up.
Baseline characteristics
Table 3 demonstrates the incidence of agreement between respondents who participated in all FU rounds with their child (n=24) and those who did not participate in any FU round (n=7). The vast majority of both groups wanted to contribute to knowledge regarding both obesity and fertility (table 3). Women who participated with their child during all FU rounds felt more involved in the FU as compared with those women who did not participate in any FU round (95.8% vs 14.3%, respectively, p<0.001). In addition, women who participated with their child in all FU rounds agreed that the way the FU study was introduced was good as compared with women who did not previously participate (91.7% vs 28.6% respectively, p=0.002). Respondents who did not participate in any child FU data collection round would have appreciated it if the plan for the FU would have been clearer at the start of the RCT and agreed more often that they would have been more likely to participate if someone familiar from the RCT would have introduced the FU, as compared with women who participated in all FU rounds (table 3). In addition, respondents who did not participate in any child FU round agreed more often that the subject of the research has to be something they personally find interesting. Almost all respondents who participated in all FU rounds agreed that the importance of the FU was clear (95.8%) as compared with 42.9% of the respondents who did not participate in any child FU round.
Agreement between respondents who participated in all FU rounds and respondents who did not participate in any FU round
FU round specific questions
Table 4 demonstrates the agreement between participants and non-participants per FU round. Overall, women who participated with their child during any FU round agreed more often that participation felt like a health check for their child as compared with non-participants. This difference increased in subsequent FU rounds, ranging from 55.1% and 38.9% between participants and non-participants in the health questionnaire to 68.3% and 28% in the cardiac assessment, respectively.
Agreement between participants and non-participants per FU round
In the health questionnaire, participants and non-participants did not differ significantly on statements, including if the questionnaire took too much time (16.3% vs 11.2%, respectively), if the questionnaire was too burdensome for their child (4.2% vs 11.2%) or if they believed that their child was too young to participate (20.4% vs 11.1%). Participants and non-participants of the physical examination or cardiac assessment round did differ on these statements. Respondents who participated in these FU rounds let the decision of participation more often fully depend on their child (39.4% for the physical examination and 52.5% for the cardiac assessment) as compared with non-participants (17.7% for the physical examination and 24% for the cardiac assessment).
Non-participants of the physical examination or cardiac assessment agreed more often that the research visit was too burdensome for their child (24.2% vs 3% for the physical examination and 37.5% vs 0% for the cardiac assessment) and took too much time (17.7% vs 3.1% for the physical examination and 25% vs 2.4% for the cardiac assessment) and they felt like their child was too young to participate as compared with participants (38.3% vs 6.1% for the physical examination and 52% vs 2.4% for the cardiac assessment) (table 4).
Sensitivity analysis
Online supplemental table 1 demonstrates the differences between respondents that participated in our study and all eligible non-respondents. Respondents of our study were older as compared with non-respondents (30.1 years (SD: 3.9 years) vs 28.8 years (SD: 4.6 years), respectively, p=0.05) and their children had a higher birth weight (3506.2 g (SD: 655.5 g) vs 3325.5 g (SD: 568.8 g), respectively, p=0.04).
Discussion
We sought to determine which factors contribute to the decision of mothers to participate with their child in FU examinations after participation in an RCT prior to conception. We found that all women who had been invited for FU of their child wanted to contribute to knowledge of the research topic. Women who participated in all rounds of data collection with their child felt more involved in the study compared with those who did not participate. In addition, women who participated with their child in the physical examination or cardiac assessment more often perceived participation as a health check for their child and let their child decide to participate as compared with those who did not participate. This suggests that important reasons for participating in FU research are feeling involved, perceiving the FU as a health check for their child and actively involving their child in the decision to participate.
In pregnant women anticipating to participate in a birth cohort study, altruism and health-related motivations are important factors for participation in research.28 29 In our study, both participants and non-participants wanted to contribute to knowledge of the research topic. In addition, half of the respondents that participated in all FU rounds with their child agreed that it is important that the research topic is something that they find personally interesting, implying altruism might not be the only driving factor for participation in FU research of their child. Perceiving the FU as a health check for their child seemed to positively influence the decision for participation. This is in line with previous research, demonstrating that participation in longitudinal research was not mainly driven by altruism as expected beforehand, but by the perceived benefits during the FU visit, such as the medical care.30 Barnett et al assessed maternal experience of participation in FU research with children after participation in a longitudinal cohort study during pregnancy.31 They identified health improvements in children as a significant motivator for mothers to remain in the study after their child was born.31 In addition, Garg et al identified perceived health benefits, regular monitoring of their child and a gain in health-related knowledge as important incentives for mothers when participating in research with their children.29 Patients seeking fertility care considered the safety of the assisted reproductive technique, which includes long-term outcomes in their unborn children, the most important research topic.32 Therefore, we believe it is important to emphasise perceived healthcare benefits to women participating in FU research for their child.
In our study, respondents who participated in all FU rounds felt more involved as compared with non-participants. Previous research exploring reasons for participation in longitudinal health studies demonstrated that a sense of loyalty and membership is positively associated with participation.30 Studies that involved patients in the study design process have higher participation rates,33 and the findings are more readily translated into clinical practice.34 Non-participants would have been more inclined to participate if the FU would have been introduced at inclusion of the RCT, and if the health outcomes assessed in FU would be relevant to them. This is in line with studies assessing the impact of patient and public involvement on enrolment and retention studies. These studies found that patient involvement in setting up studies, for example, in the direction and priorities of studies, leads to more active and involved participants.35–37 This might also lead to a clearer understanding of the importance of the FU, something we found to be two times as high among participants as compared with non-participants. Therefore, we believe that patient involvement in priority setting, designing and execution of research will lead to a higher participation rate and facilitate implementation of knowledge gained by research into practice.38
Women who participated with their child in the FU consisting of physical examination or cardiac assessment more often allowed their child to decide if she/he wanted to participate. Thus, when inviting women with their children for FU research, it is important to encourage women to actively involve their child in the decision of participation, and to ensure appropriate information for the child, such as a separate invitation letter. A review on the participation of children in research identified that only 15% of research claiming to involve children in the design of studies actually involved them in the decision to participate in research,39 even though involving children in all aspects of research leads to more committed and involved participants.40 When designing an FU of RCTs before or during pregnancy, representative children should be involved to ensure that the research appeals to children.
The FU rates in the data collection rounds were low. The FU rate of the physical examination was significantly lower than the same protocol that was carried out by the same team during the FU of an RCT of assisted reproduction techniques in couples with unexplained or mild male subfertility (The INeS study) (33% vs 57%, respectively).41 Importantly, although both FU studies were carried out in the same way, in the same time period, and by the same team, the participation rates differed. Both studies investigated infertile couples aiming to conceive, but the current study only included women who also were overweight and obese, while the INeS trial did not. Moreover, the lifestyle intervention was aimed at weight loss rather than conception, while the INeS study randomised women to different fertility treatments. Although the link between obesity and subfertility was known to most participants in our study, women included in our RCT did not seek medical care for their weight even though the intervention offered to these women consisted of lifestyle counselling. We hypothesise that offering a lifestyle intervention for an unfulfilled wish to become pregnant might have led to a feeling of disconnect between their medical problem and the treatment offered. These factors could have played a role in the reduced willingness to participate in our FU.
Respondents filling out our questionnaire reported not feeling they were being stigmatised due to their weight. However, this may have been different for non-responding women. Previous research has demonstrated that women with obesity are often faced with weight stigma.42 43 Raising the topic of weight by healthcare providers requires a sensitive and respectful approach, using neutral terminology (eg, ‘weight’ and ‘body mass index’ instead of ‘obese’) and preferably asking women about their language preferences.44 Moreover, healthcare providers should not make assumptions about diet, activity levels, motivations and perceived difficulties.45 Women with obesity contemplating a pregnancy are often not aware of the detrimental consequences of maternal obesity on their future child.46–49 However, once they are made aware of these consequences they are often willing to improve their health and postpone their wish to conceive in order to make lifestyle changes.50 Unfortunately, if information about lifestyle is provided by a healthcare professional, it is often unclear and inconsistent, which makes women perceive the message as unimportant.51 Taken together, healthcare providers working with women with obesity contemplating a pregnancy need to be adequately informed regarding the benefits of a healthy lifestyle during pregnancy and educated to address this topic in a non-judgmental manner.45 46 In addition, the social context has a great influence on lifestyle and should be recognised when implementing a lifestyle intervention in women with obesity.52 Furthermore, if the social context is included, women feel supported in daily life and perceive the implementation of a healthy lifestyle during pregnancy as a shared responsibility instead of an individual responsibility.51
There are limitations to our study. First, only 32% of all eligible mothers and children participated in this study, making our results prone to selection bias. If we compare women who participated in our study with eligible non-respondents, we find that respondents were older and gave birth to children with a higher birth weight (online supplemental table 1). This participation bias is often reported in FU of birth cohorts.53 54 However, the differences were small and several extreme low birthweight children in the non-respondent group were responsible for the significant difference in birth weight (data not shown). We found no other differences between respondents and eligible non-respondents. Therefore, we believe our results are representative of the entire group of participants and the findings are likely to reflect true reasons to participate in FU of children after maternal participation in an RCT. Second, our study includes women with obesity and infertility, which may limit the generalisability of our results. Women with obesity contemplating a pregnancy are not often in contact with healthcare providers, unless they experience problems to conceive.55 As a result, trials assessing a preconception lifestyle intervention in women with obesity often include women that present with fertility issues.55 However, we expect the motivation to participate in a study that stimulates a healthy lifestyle to optimise child’s health is independent of a women’s fertility status. Therefore, we believe that our findings also apply to other women.
Conclusion
When designing an FU in children after maternal participation in an RCT of an intervention before or during pregnancy, loss-to-FU might be limited by emphasising the possible perceived benefits of participation, such as a health check for their child, and to encourage women to actively involve the child in the decision of participation. In addition, it is important to actively involve women and representative children in the design of the FU study to stimulate the sense of involvement and increase understanding of the importance of the FU, which seems to increase participation rates. Implementing these factors could prevent loss-to-FU and eventually help to assess causality between early life and later health.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. Please contact authors for information on data availability.
Ethics statements
Patient consent for publication
Ethics approval
The Medical Ethics Committee of the UMC Groningen deemed that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study (METc 2019/221) and official approval was not required. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to thank Casey Johnson for his assistance reviewing this manuscript for grammar.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Groen62H
Contributors TdH and AvD designed the research protocol. TdH was responsible for safely storing all data, extracting and analysing the data and writing the article. AH, HG and TJR all carefully reviewed the article. AH, HG, TJR and AvD were involved in the set-up of the original intervention study and follow-up study. All authors provided intellectual input and were involved in the writing of the article. TdH, AvD and TJR acted as guarantors.
Funding This work was supported by a grant of the Dutch Heart Foundation (grant number: 2013T085) and a Postdoc Stipend of Amsterdam Reproduction and Development. The initial LIFEstyle trial was supported by a grant from ZonMW, the Dutch Organization for Health Research and Development (grant number: 120620027).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.