Article Text
Abstract
Introduction Antenatal care addressing alcohol consumption during pregnancy is not routinely delivered in maternity services. Although a number of implementation trials have reported significant increases in such care, the majority of women still did not receive all recommended care elements, and improvements dissipated over time. This study aims to assess the effectiveness of an iteratively developed and delivered implementation support package in: (1) increasing the proportion of pregnant women who receive antenatal care addressing alcohol consumption and (2) sustaining the rate of care over time.
Methods and analysis A stepped-wedge cluster trial will be conducted as a second phase of a previous trial. All public maternity services within three sectors of a local health district in Australia will receive an implementation support package that was developed based on an assessment of outcomes and learnings following the initial trial. The package will consist of evidence-based strategies to support increases in care provision (remind clinicians; facilitation; conduct educational meetings) and sustainment (develop a formal implementation blueprint; purposely re-examine the implementation; conduct ongoing training). Measurement of outcomes will occur via surveys with women who attend antenatal appointments each week. Primary outcomes will be the proportion of women who report being asked about alcohol consumption at subsequent antenatal appointments; and receiving complete care (advice and referral) relative to alcohol risk at initial and subsequent antenatal appointments. Economic and process evaluation measures will also be reported.
Ethics and dissemination Ethical approval was obtained through the Hunter New England (16/11/16/4.07, 16/10/19/5.15) and University of Newcastle Human Research Ethics Committees (H-2017-0032, H-2016-0422) and the Aboriginal Health and Medical Research Council (1236/16). Trial findings will be disseminated to health service decision makers to inform the feasibility of conducting additional cycles to further improve antenatal care addressing alcohol consumption as well as at scientific conferences and in peer-reviewed journals.
Trial registration number Australian and New Zealand Clinical Trials Registry (ACTRN12622000295741).
- Quality in health care
- Organisational development
- Protocols & guidelines
- PUBLIC HEALTH
- OBSTETRICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This will be the first controlled trial to evaluate the effectiveness of an iteratively developed and delivered implementation support package in increasing and sustaining the routine provision of antenatal care addressing alcohol consumption during pregnancy.
The implementation support package was developed based on an assessment of outcomes and learnings following the initial trial and consists of evidence-based implementation and sustainability strategies.
The stepped-wedge cluster study design is appropriate for implementation trials that deliver implementation support at a service level and offers pragmatic and scientific strengths to the study.
Data will be collected through surveys of women who recently attended an antenatal appointment, which is subject to less response bias than health-professional self-report of clinical adherence and provides complete outcome data unlike medical records.
The order in which the sectors receive the implementation support package will be non-randomised.
Introduction
Alcohol consumption during pregnancy can lead to adverse obstetric (risk of placental abruption, miscarriage and preterm birth1–3) and child outcomes (birth defects, developmental delays and fetal alcohol spectrum disorder4–6). Many countries have released guidelines that recommend no alcohol consumption in pregnancy.7 Despite such recommendations, the global prevalence of alcohol consumption during pregnancy has been estimated at 10%, with higher prevalence estimates reported in a number of high-income countries, including Ireland (60%), Denmark (46%), the UK (41%) and Australia (36%).8
Systematic review evidence shows that pregnant women who receive brief psychosocial interventions from healthcare providers are more than two times as likely not to consume alcohol during pregnancy (OR: 2.31; 95% CI 1.61 to 3.32; p<0.001).9 Consistent with such evidence, clinical guidelines recommend that all women at initial and subsequent antenatal appointments receive: (1) assessment of alcohol consumption; (2) advice not to consume alcohol and discussion of the risks and (3) referral to specialist services for further assessment, diagnosis of alcohol use disorders and treatment if required.10 11 Public maternity services are a critical setting for these guidelines to be implemented as they provide care to the majority of pregnant women in many countries, including Australia.12 13 However, clinician adherence to the guideline recommendations in these services is low (assessment: 42%–64%14–16; advice: 11%–35%16 17; referral: 10%–50%16 18 and all guideline elements: 4%–28%16).
Two controlled trials to date have tested the effectiveness of implementation strategies in increasing the provision of antenatal care addressing alcohol consumption during pregnancy.19 20 The first trial conducted in 2013 with four Italian Obstetrics and Gynaecology Units found that training significantly increased the proportion of pregnant women who received guideline consistent alcohol advice from their midwife (intervention: 53% vs control: 20%; Risk Ratio: 2.66; 95% CI 1.27 to 5.56).19 The second trial, conducted with all public maternity services in three sectors of a single local health district in Australia between 2017 and 2020, found that an implementation support package consisting of seven evidence-based strategies significantly increased the proportion of pregnant women who reported receipt of: assessment of alcohol consumption via the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) tool (pre-implementation: 28.4%; post implementation: 40.6%; OR: 2.63; 95% CI 2.26 to 3.05; p<0.001); advice not to consume alcohol and discussion of the potential risks (pre-implementation: 18.7%; post implementation: 26.7%; OR: 2.07; 95% CI 1.78 to 2.41; p<0.001); complete care (advice and referral) relative to women’s alcohol risk level (pre-implementation: 18.5%; post implementation: 26.6%; OR: 2.10; 95% CI 1.80 to 2.44; p<0.001); and all guideline elements (assessment, advice and referral) relative to alcohol risk level (pre-implementation: 12.6%; post implementation: 19.4%; OR: 2.32; 95% CI 1.94 to 2.76; p<0.001).20 The effect sizes in both studies were at the upper end of implementation trial outcomes as reported in Cochrane systematic reviews.21–30 However, half or fewer reported receipt of the recommended care elements after implementation support, leaving many women without the intended benefits of the clinical guidelines. Such a finding is consistent with the clinical practice change literature generally, which indicates that despite significant effect sizes in trials, the interventions do not result in the majority of patients receiving guideline recommended care.
Improvements in healthcare are rarely breakthrough in nature, rather they tend to occur gradually as new evidence is generated and applied.31 Public health approaches to addressing health risks recognise that multiple steps are required for improvements to occur (eg, defining the problem, understanding the determinants of the problem, designing strategies and implementing/evaluating strategies) and that often such steps need to be repeated as the evidence-base is built over time.32 This is also evident in quality improvement approaches used in healthcare settings to improve processes, safety and patient care outcomes.33 In such approaches, systematic modifications are iteratively made until stakeholder defined outcomes are met and/or sustained practices are achieved.34 Implementation trials that have used such approaches have demonstrated improvements in the proportion of patients receiving evidence-based interventions, including smoking cessation counselling in general practice35 and HIV viral load monitoring in antenatal care.36
There has been one study to date that has used an iterative improvement approach to increase the proportion of pregnant women receiving antenatal care addressing alcohol consumption during pregnancy.37 Fifty Australian primary healthcare centres participated in four cycles of continuous quality improvement between 2007 and 2012 to improve pregnancy care for Aboriginal and Torres Strait Islander women. At the beginning of each cycle, a systems assessment and audit of patient records was conducted to identify opportunities for improvement. A longitudinal analysis of 2220 pregnancy records found that effects continued to increase for alcohol screening (cycle 1 OR: 2.6; 95% CI 2.0 to 3.5; cycle 4 OR: 3.9; 95% CI 2.2 to 7.1) and brief counselling (cycle 1 OR: 2.8; 95% CI 1.7 to 4.5; cycle 4 OR: 6.7; 95% CI 2.3 to 20.0) over the four cycles compared with baseline. Over the duration of the study, care provision increased by 18% for screening (65%–83%) and 20% for counselling (51%–71%).37 The study, however, was non-controlled and the generalisability of results to the public hospital maternity service setting and non-Indigenous populations is unknown.
A further limitation of successful controlled implementation trials generally, is that observed effect sizes do not persist.38 For example, in the Australian controlled trial described earlier, a time series analysis that explored the rate of weekly change in recommended alcohol care delivery outcomes for 17 months after the implementation found significant decreases in both assessment (−0.66%; 95% CI −1.1 to –0.26; p=0.002) and complete care (−0.64%; 95% CI −1.1 to –0.22; p=0.003).20 No specific sustainability strategies were incorporated into the implementation support package delivered in the trial. This suggests that factors that commonly impede sustainment of care delivery change may not have been sufficiently addressed by the trial implementation support package39 and that specific sustainability strategies may be required to ensure achieved effect sizes are maintained.40 A limited number of studies have tested the effect of sustainability strategies in maintaining improvements in evidence-based interventions in maternity service settings,41 42 with none specific to alcohol care. Such studies have found maintenance of workforce skills through ongoing training and mentoring opportunities, leadership buy-in and reviews of progress against improvement goals have sustained improvements in a range of antenatal care practices for periods between 1 and 5 years.41 42
The need to find effective strategies to both improve and sustain the routine provision of antenatal care addressing alcohol consumption during pregnancy remains. Given the potential of an iterative care delivery improvement approach and the inclusion of specific sustainment strategies to achieve this, and the limited research to date testing the effectiveness of such approaches, an implementation trial will be conducted to assess the effectiveness of an implementation support package including such approaches in: (1) increasing the proportion of pregnant women who receive guideline recommended antenatal care addressing alcohol consumption and (2) sustaining the rate of care over time.
Methods and analysis
The study methods were developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (online supplemental additional file 1).
Supplemental material
Study design and setting
This trial follows on from a randomised stepped-wedge cluster trial that was conducted in public maternity services in three sectors within the Hunter New England Local Health District (HNELHD), New South Wales, Australia, between 2017 and 2020 (referred as the ‘initial trial’ from this point forward).20 This trial will also use a stepped-wedge cluster study design and be conducted with the same services that participated in the initial trial to further enhance care delivery. The stepped-wedge cluster study design provides scientific and pragmatic advantages for conducting implementations trials in health settings, including: providing the same level of evidence as standard parallel cluster controlled trials; addressing the practical difficulty of recruiting enough equivalent maternity services required for parallel cluster controlled trials and increasing study efficiency by using each group as its own control.29 43
As shown in figure 1, continuous cross-sectional outcome data will be collected with weekly random samples of pregnant women who have recently attended an antenatal appointment with a participating maternity service. Delivery of a 3-month implementation support package will occur sequentially at the three sectors, which will provide outcome data periods of variable lengths for each sector. As per the initial trial, the intervention effect for aim one will be determined by comparing the overall proportion of women who report recommended care between pre-implementation and post implementation periods for the three sectors combined. This will be assessed 6 months after implementation completion in the last sector. For aim two, an additional 4 months of post implementation data will be collected for all three sectors to allow for a more prolonged assessment of care delivery sustainment. The primary outcomes will be reanalysed using a multiple baseline design to explore the rate of change over time as the measure of sustainment.
Data collection and study design.
The study is being conducted in three geographically and administratively distinct sectors. The maternity services within these sectors provide antenatal care to 6100 women annually (70% of births in the district). Sectors 1 and 2 are located in regional/rural areas (1200 and 600 births, respectively) and sector 3 in a major city (4300 births per annum).44
Participant blinding
Research staff collecting outcome data will be blind to the order in which the three sectors receive the implementation support package. Participants will not be informed of the experimental nature of the implementation rollout and therefore will be blind to the stage of the study in the maternity service they attend. Given that maternity service staff will receive the implementation support package, they will be aware when their service is in the implementation period.
Participant eligibility and recruitment
Maternity services and staff
As per the initial trial, all maternity services within the three sectors will receive the implementation support package. These services include: midwifery led services and clinics; medical led clinics; and Aboriginal Maternal Infant Health Services (AMIHS). All antenatal care providers in these services (midwifery and medical staff and Aboriginal Health Workers) will be eligible to receive implementation support. This trial will also extend to maternity service staff who are in positions that support the ongoing availability and usage of the implementation strategies (maternity unit managers, administrative staff and clinical midwifery educators (CMEs)). All antenatal care providers will be invited to participate in surveys prior to implementation. All maternity service staff targeted to receive the implementation support package will be invited to participate in post implementation surveys.
Pregnant women
All women who attend an antenatal appointment at a participating maternity service have the potential to receive assessment and care addressing alcohol consumption as part of usual antenatal care. Women are eligible to participate in data collection following attendance at their: (1) initial antenatal appointment or (2) 27–28 weeks gestation appointment or (3) 35–36 weeks gestation appointment. Further eligibility criteria: aged 18 years or older; 12–37 weeks gestation; sufficient level of English to complete the survey and mentally and physically capable of completing the survey. Ineligibility criteria: receiving the majority of antenatal care through a private provider; given birth; negative pregnancy outcome; selected to participate in the data collection in the preceding 4 weeks or previously declined participation in the surveys. The number and characteristics of women deemed ineligible will be reported.
Each week, all eligible women from sector 1 and sector 2 will be sampled. For sector 3, a random sample of eligible women will be generated via a computerised random-number generator by members of the research team not involved in delivering care to women. All women will be sampled in sector 1 and sector 2 given the smaller number of women who attend these services. To enhance representativeness of the data collected, all women who are identified in the medical record data as being of Aboriginal and/or Torres Strait Islander origin (the term Aboriginal will be used from this point) and women who are attending or enrolled to attend an AMIHS will also be selected.
All women will receive a study information flyer in their usual antenatal information packs. Selected women will be sent a participant information statement outlining the purpose of the survey 1 week prior to receiving a telephone call inviting participation in the survey. As per advice from Aboriginal stakeholders regarding a culturally appropriate recruitment method for Aboriginal women, Aboriginal women and/or women attending or enrolled to attend an AMIHS will be contacted by text message 3 days after the information statement is sent and invited to participate in the survey via telephone or online modes. If no response is received, a telephone call will be attempted 4 days later. On the day that a woman is to be contacted to invite participation, medical record data will be checked and any women who have given birth or had a negative pregnancy outcome will be deemed ineligible.
Model of care and implementation support package
Evidence-based model of antenatal care
The evidence9 45 and guideline-based10 11 model of antenatal care found to be acceptable to Aboriginal (95%) and non-Aboriginal pregnant women (99%) and to antenatal care providers (78%–91%) in the initial trial20 will be delivered to all pregnant women attending an initial antenatal appointment, 27–29 weeks and 35–37 weeks antenatal appointment (figure 2). The model of care is based on the Screening, Brief Intervention and Referral to Treatment public health approach to the management of substance abuse46 and consists of three key elements:
Assess: assess all women’s alcohol consumption using the AUDIT-C tool.47 Women’s responses will be used to assign a risk of harm category: no risk (AUDIT-C score=0); low risk (AUDIT-C score=1–2); medium risk (AUDIT-C score=3–4) and high risk (AUDIT-C score=5+).
Advise: advise all women not to consume alcohol during pregnancy and discuss the potential risks.
Refer: offer women at medium risk a referral to the free government funded Get Healthy in Pregnancy telephone-based coaching service, which supports women to make positive changes to their health, including abstaining from alcohol during pregnancy.48 Also offer Aboriginal women at medium risk a referral to counselling services delivered through local Aboriginal Community Controlled Health Services. Offer women at high risk a referral to HNELHD Drug and Alcohol Clinical Services, which provide further assessment and diagnosis of alcohol use disorders, brief intervention, treatment and withdrawal support as clinically indicated.
Evidence-based model of antenatal care recommended for provision at the initial and subsequent antenatal appointments. AUDIT-C, Alcohol Use Disorders Identification Test- Consumption.
Implementation support package
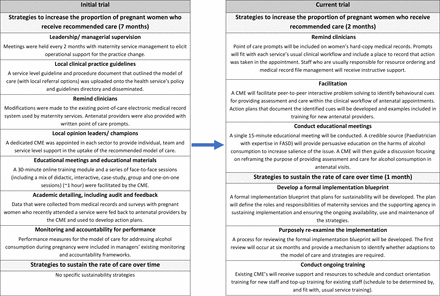
The initial trial delivered a comprehensive implementation support package that sought to increase the proportion of pregnant women receiving all elements of the model of antenatal care. As the majority of pregnant women in that trial (89.0%) were found to have been asked about alcohol consumption at the initial antenatal appointment, the implementation support package in this trial will not specifically seek to improve this care element.20 The trial implementation support package will incorporate strategies that specifically address its two aims based on an assessment of outcomes and learnings from the initial trial. As per implementation science recommendations,49 the support package will be targeted to the specific barriers and context of the local maternity service setting. See figure 3 for a description of the implementation support packages used in the initial trial and those proposed for this trial, and figure 4 for the logic model of this trial.
Implementation support packages used in initial and current trial. CME, clinical midwifery educator. FASD, fetal alcohol spectrum disorder.
Logic model.
Strategies to increase the proportion of pregnant women who receive antenatal care addressing alcohol consumption
In the initial trial, formative research using the theoretical domains framework (TDF)50 51 was conducted to comprehensively assess a range of barriers to implementing the recommended model of care. To address change in barriers (or their relative importance) over time, surveys were conducted with antenatal providers in the three sectors following completion of the trial to identify the highest priority barrier/s to delivering two care elements (assessment at subsequent antenatal appointments and advice discussion) using a best-worst scaling method.52 Two priority barriers were found: (1) forgetting and (2) not believing there is a need to provide alcohol focused care to all women. Forgetting had been identified as a barrier in the initial formative research using the TDF, but its relative importance among all identified barriers had not been ascertained due to the survey method used. Not believing in the need to provide alcohol focused care to all women was not previously identified.
Similar to the initial trial, the priority barriers were defined in terms of the TDF50 51 and Capacity, Opportunity, Motivation-Behaviours model53 and mapped to intervention functions and behaviour change techniques (BCTs) using the behaviour change wheel.53 Process evaluation data collected in the initial trial was used to inform the delivery of the implementation strategies. Components of strategies that had achieved high level/wide reach and were rated as acceptable and appropriate by antenatal providers were incorporated into the delivery of strategies. Clinical representatives and Aboriginal health staff provided expertise to finalise the strategies and embed cultural appropriateness for Aboriginal women (see online supplemental additional file 2 for development of implementation strategies).
Supplemental material
Based on the above intervention development methods, the following strategies, defined according to the Expert Recommendations for Implementing Change taxonomy,54 will be delivered: remind clinicians; facilitation and conduct educational meetings. The initial trial implemented reminders as a strategy built into the electronic medical record system. This strategy did not reach all maternity service types (eg, home visits) and profession types (eg, some medical and Aboriginal Health staff did not use the electronic medical record system). To address this, stickers for hard-copy medical records were implemented reactively during the initial trial and were subsequently rated as the most useful resource by antenatal providers (range: 72%–85%). The stickers, were primarily designed and used to record care provision (rather than prompt) and only included assessment of alcohol consumption (not advice or referral). Their availability and usage were also dependent on administrative staff who were not provided with implementation support. These two issues will be addressed in the remind clinicians strategy used in this trial.
Two additional implementation strategies (facilitation; conduct educational meetings) will involve BCTs not used in the initial trial. A CME will deliver peer-to-peer facilitation to support antenatal providers identify behavioural cues for providing assessment and care in the clinical workflow of subsequent antenatal appointments. A CME will conduct educational meetings that will use a credible source to deliver persuasive information on the harms of alcohol consumption during pregnancy and provide new perspective on the purpose of assessment of alcohol consumption at subsequent appointments and having advice discussions with all women using framing/reframing techniques.53
Strategies to sustain the rate of care over time
A process for developing strategies to sustain the rate of care over time was undertaken guided by principles of the dynamic sustainability framework (DSF).55 The DSF seeks to address change in three areas: the evidence-based intervention (eg, mode of delivery); practice setting (eg, information systems, training and staffing) and ecological systems (eg, policies). To determine the changes that had occurred in each of these areas since the initial trial, consultations were undertaken with clinical representatives, and audits of antenatal schedules, training records, staffing rosters, information systems, and resource and policy databases were conducted.
Although it was found that there had been a marked increase in antenatal appointments delivered via telehealth in response to the COVID-19 pandemic, telehealth care delivery guidelines included alcohol care being delivered irrespective of appointment mode. An assessment of systems and resources available to support care provision indicated that the majority of strategies implemented in the initial trial were still fully or partially available. An assessment of workforce turnover indicated that almost half of the current antenatal care workforce was not employed at the time of the initial trial and almost half of these new staff had not completed any of the training made available through the initial trial strategy. In addition, no formal process that defined the roles and responsibilities of specific groups or staff in ensuring the ongoing availability and use of supporting systems and resources, nor a formal process for identifying when adaptions to the model of care and implementation strategies may be required to address changes in circumstances. To address these factors, three strategies were selected based on the sustainability literature and in consultation with experts in the field: develop a formal implementation blueprint; purposely re-examine the implementation and conduct ongoing training54 (see online supplemental additional file 3 for development of strategies).
Supplemental material
Implementation delivery timeline
The implementation support package will be delivered in each of the sectors sequentially for a period of 3 months (see figure 1). Strategies aimed at increasing the proportion of women who receive antenatal care addressing alcohol consumption will be delivered in the first 2 months of the implementation. Strategies aimed at sustaining the rate of care will be developed, agreed to and implemented in the third month. Given the focus on embedding sustainability, the implementation support package has the potential to continue supporting care provision following the 3-month implementation.
Control and contamination
Usual care
In the pre-implementation data collection phase for each of the three sectors, usual antenatal care for addressing alcohol consumption during pregnancy will be provided. Strategies available to support care provision include: national and local clinical practice guidelines; electronic medical record prompts; online education module and performance data entered into the health service’s monitoring system quarterly. Care provision is likely to vary by maternity service and clinician.
Potential for contamination
As the research team will control implementation delivery, the implementation support package will not be accessible to maternity services during the pre-implementation (control) phase.
Patient and public involvement
Pregnant women’s acceptability of the model of care was considered in the development of the evidence-based intervention for this trial. Antenatal care provider’s feedback on the initial implementation support package and new consultations with clinical representatives informed the iterative development of this trial’s support package. Consultations with Aboriginal health staff were undertaken to embed cultural appropriateness for Aboriginal women across all components of the trial. A Cultural Review Group containing only Aboriginal members, including health service and community representatives, will review all dissemination products.
Measures
Primary trial outcomes
The proportion of all pregnant women who report:
Being asked about alcohol consumption at subsequent antenatal visits.
Receiving complete care (advice and referral) relative to level of alcohol risk at subsequent antenatal visits.
Receiving complete care (advice and referral) relative to level of alcohol risk at the initial antenatal visit.
Process measures
Fidelity, penetration/reach and acceptability will be assessed in accordance with the implementation evaluation framework specified by Proctor et al.56 Measures to assess penetration/reach will include the proportion of eligible staff who were exposed to each of the strategies. Acceptability of the strategies will be measured from the perspective of maternity staff. Sustainment at the provider and inner-context levels will be measured from the perspective of maternity staff using the three-item Provider REport of Sustainment Scale.57 Changes occurring at the outer contextual level (eg, social, political and economic factors) that may influence practices will be monitored and reported.
Within-trial economic analyses
A trial-based cost-effectiveness analysis will calculate the incremental cost per unit change in the primary trial outcomes and cost-consequence analysis will disaggregate results by sector. To assess the affordability of sustaining care over time within the resource and budget constraints of the health service, a budget impact analysis will also be conducted. All analyses will be conducted and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards publication guidelines and good reporting practices guidelines.58
Data collection procedures
Primary outcome measures
Telephone contact will be attempted with sampled women up to 10 times over a 2-week period in order to elicit consent and completion of the survey. Women who decline participation in the telephone survey will be offered the online survey. Aboriginal women and/or women attending or enrolled to attend AMIHS will be offered the choice of telephone or online mode at first contact. The telephone survey will be computer assisted and be conducted by trained female interviewers. The questions and response options will be identical in the telephone and online surveys. All data collected will be recorded in the online Research Electronic Data Capture (REDCap).59 60
Process measures
Process measures will be collected through surveys with maternity staff and project management logs. Surveys of maternity service staff will be conducted pre-implementation (sustainment only) and post implementation in each sector (penetration/reach, acceptability and sustainment). Eligible staff will be sent a link to an online survey via email as well as given the option to complete the survey on tablet computers or pen and paper during regular clinic meetings. Additional process data will be collected by project staff during the implementation period and recorded in project management logs.
Costs
Resource use associated with the implementation support package will be prospectively identified, measured and valued using a cost capture template to be developed in REDCap.59 60 Implementation resources are expected to include labour and materials to support maternity service staff. Costs associated with implementation will be recorded separately from those used for sustainability.
Sample size and power calculations
Assuming that 225 women will complete a survey per month (approximately 150 for subsequent antenatal visit time points and 75 for the initial antenatal visit time point), we will have 80% power to detect an absolute increase of approximately (1) 15% in being asked about alcohol consumption at subsequent antenatal visits (baseline prevalence of 42%); (2) 13% in complete care at subsequent antenatal visits (baseline prevalence of 23%) and (3) 21% in complete care at initial antenatal visits (baseline prevalence of 45%). This is assuming an Intraclass Correlation Coefficient of 0.01 and an alpha level of 1.67% (Bonferroni adjusted for the three primary outcomes).
Statistical analyses
To address the first aim, pre–post differences in the proportion of women reporting receipt of care for each of the three primary outcomes will be compared using generalised linear models with a binomial distribution and logit link function. These models will compare the odds of receiving care at post implementation versus pre-implementation. Each model will contain a term for period (pre-implementation or post implementation), sector (1, 2, 3), antenatal visit for the outcomes on subsequent antenatal visits (28 weeks gestation, 36 weeks gestation) and time (in months). An alpha level of 1.67% will be used to determine statistical significance. The OR and 95% confidence limit from the term for period will be presented as the intervention effect.
For the second aim, segmented regression within an interrupted time-series framework will be used to assess women’s receipt of care over time, and whether this improves and sustains following the delivery of the implementation support package. These analyses will be on the same three primary outcomes assessed in the pre–post difference analyses and will be conducted separately for each of the three sectors. Replication of findings across the three sectors will provide greater confidence in the intervention effect.61 Three segments will be specified in each segmented regression, one for each of the study phases (ie, pre-implementation, implementation and post implementation). The rate of change in the receipt of care will be estimated for each of the three segments.
Exploratory secondary analyses will also be conducted to examine trial outcomes relative to initial trial findings, including a comparison of the proportion of pregnant women receiving guideline recommended care and rate of change per month of implementation support.
Research trial governance
The conduct of the trial will be overseen by an advisory group consisting of researchers, practitioners and clinical experts with expertise related to alcohol consumption during pregnancy, clinical practice change, sustainability, maternity services, Aboriginal heath and health economics. A project team consisting of research staff and a project dedicated CME will operationalise all components of the trial according to study protocol.
Aboriginal cultural governance
Cultural governance will be embedded across the trial to be inclusive of Aboriginal people’s perspective. Aboriginal cultural task groups that are led by an Aboriginal project team member will provide guidance on the delivery of the implementation support package. A Cultural Review Group containing only Aboriginal members will review all dissemination products.
Trial status
Recruitment of Sector One will commence April 2022 and recruitment of the last Sector will be completed in December 2022. Data collection will be completed by December 2023 and data analysis will commence January 2024.
Ethics and dissemination
Ethical approval was obtained through the Hunter New England Human Research Ethics Committee (16/11/16/4.07, 16/10/19/5.15); the University of Newcastle Human Research Ethics Committee (H-2017-0032, H-2016-0422) and the Aboriginal Health and Medical Research Council (1236/16). Any modifications to the protocol will be submitted to the above-mentioned ethics committees for approval prior to implementation. There are no predetermined criteria for trial discontinuation. Any unforeseen adverse events will be reported to the Hunter New England Human Research Ethics Committee (primary approval committee). The trial registry will be updated with any protocol modifications and any deviations from the original protocol will be reported.
Participation in the women and staff surveys will be voluntary. Potential participants will receive information about the study prior to providing verbal informed consent for surveys conducted via phone or written consent for surveys completed via online/pen paper modes. Women will have the opportunity to decline participation at any point, including after receiving the study information flyer or participant letter; at the time of the telephone call or text message; or partway through survey completion. Staff will also have the opportunity to decline participation at any point. A data management protocol that was developed and approved by the advisory group for the initial trial will be used in this trial. All data will be stored securely as per the requirements of the approving ethics committees and confidential identifying participant information will not be linked to survey responses. Data will only be accessible to the project team.
Trial findings will be disseminated to health service decision makers to inform the feasibility of conducting additional cycles to further improve antenatal care addressing alcohol consumption. Findings will also inform the use of iterative improvement approaches for other antenatal care guidelines in maternity services that have low adherence. Trial findings will be disseminated to key stakeholder groups, including clinical representatives and Aboriginal partners and community organisations. Finally, outcomes will be disseminated through peer-reviewed publications and at national and international conferences.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Em_Doherty, @NicoleKNathan
Contributors ED, MK, NN, AH and JW led the overall development of the research protocol and ED led the development of the manuscript. JW, LW, MK and ED contributed to the development of the rationale and background for the protocol. ED, LW, MK, NN, AH and TM contributed to the development of the implementation support package. BT facilitated the provision of cultural advice and establishment of cultural governance structures. IS contributed clinical expertise relevant to the maternity services setting. EJE, AJD and TWT contributed clinical expertise relevant to alcohol consumption in pregnancy. ED and MK contributed to the development of data collection methods generally and PR and OW contributed to the development of data collection methods specific to the cost and cost-effectiveness measures. AH and JA provided overall guidance for the study design and data analysis. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.