Article Text
Abstract
Objective To compare outcomes between O and non-O blood groups, and by modified RNA (mRNA) and adenovirus-vectored (Ad-V) vaccines.
Design Population-based cohort study.
Setting All of Ontario, Canada. Linked data sets captured clinical encounters, vaccinations and laboratory testing for SARS-CoV-2.
Participants Individuals aged 12+ years with known ABO blood group and free of SARS-CoV-2 before 15 January 2021.
Main outcomes measures The main exposure, first SARS-CoV-2 vaccination, was modelled in a time-varying manner. O and non-O blood group was known prior to vaccination. SARS-CoV-2 infection, and severe COVID-19 (hospitalisation or death), were assessed starting 14 days after vaccination, up to 27 June 2021.
Results 2 472 261 individuals were included. 1 743 916 (70.5%) had at least one vaccination, of which 24.6% were fully vaccinated. Those vaccinated were more likely to be women, older in age, residing in a higher-income area and have higher rates of certain comorbid conditions, like cancer, diabetes and hypertension. Relative to unvaccinated, after receiving their first mRNA (adjusted HR (aHR) 0.46, 95% CI 0.44 to 0.47) or Ad-V (aHR 0.49, 95% CI 0.44 to 0.54) vaccine, the risk of SARS-CoV-2 infection was lower, as was severe COVID-19 (aHR 0.29, 95% CI 0.20 to 0.43 (mRNA); aHR 0.29, 95% CI 0.26 to 0.33 (Ad-V)). Stratifying by blood group produced similar results. For example, after first mRNA vaccination, the aHR of severe COVID-19 was 0.31 (95% CI 0.27 to 0.36) among non-O blood groups, and 0.27 (95% CI 0.22 to 0.32) among O blood groups, relative to unvaccinated. Fully vaccinated individuals had the lowest risk of SARS-CoV-2 and severe COVID-19.
Conclusions SARS-CoV-2 infection and severe COVID-19 are reduced by vaccination. This effect does not vary by vaccine type or blood group, but is more pronounced among fully, than partially, vaccinated individuals.
- HAEMATOLOGY
- INFECTIOUS DISEASES
- Epidemiology
- COVID-19
- Blood bank & transfusion medicine
Data availability statement
No data are available. No additional data available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study was limited to persons who had ABO blood group testing, and who are more likely to have required blood transfusion or to have been pregnant in the past.
We did not know who had acquired natural immunity to SARS-CoV-2.
The potential for immortal time bias was mitigated by treating vaccine exposure as time varying, and by setting follow-up time to a common starting date.
The current study was largely completed prior to the emergence of the SARS-CoV-2 Delta/B.1.617 variant.
Introduction
Emergence of the SARS-CoV-2 pandemic, and COVID-19-related disease, led to rapid development of various vaccines. Efficacy was demonstrated for the modified RNA (mRNA) vaccine of the SARS-CoV-2 spike protein, to induce neutralising antibodies,1 as well as a recombinant, replication-incompetent adenovirus vector that encodes a full-length and stabilised SARS-CoV-2 spike protein (adenovirus-vectored (Ad-V)).2 Demonstrated vaccine efficacy shown from pooled data of randomised clinical trials is 95% (95% CI 94 to 95) and 80% (95% CI 56 to 93), respectively.3 Even within 14 days of receipt of a first dose, vaccine efficacy can reach 80%.4 5
It is of interest that adults with O blood group appear to be at lower risk of SARS-CoV-2 infection and COVID-19-related severe illness, compared with those with A, B and AB (ie, non-O) blood groups.6 7 Those with O blood group are identified by their anti-A and anti-B antibodies; these same antibodies may offer immunoprotection against SARS-CoV-2, as they are concomitantly produced by certain epithelial cells within the respiratory and digestive tract—prime targets for COVID-19 tissue injury.7 What is not known, however, is whether vaccinated persons with O blood group experience different rates of SARS-CoV-2 infection and COVID-19 disease than those of non-O blood group. Such information might guide vaccine type, recipient prioritisation and the need for repeat vaccination.
The current study evaluated SARS-CoV-2 infection and COVID-19 disease in a population with a universal vaccination system offered to those aged 12+ years, a high uptake of at least one vaccine8 and systematic collection of vaccination and infection data. Herein, we compared outcomes between O and non-O blood groups, and by mRNA and Ad-V vaccines.
Method
This population-based retrospective cohort study was performed in Ontario, Canada. Patient-level data sets included all hospitalisations, emergency department visits, the majority of laboratory tests for SARS-CoV-2 and all SARS-CoV-2 vaccinations administered within Ontario,9 as further detailed in online supplemental table S1.6 10 Data sets were linked using unique encoded identifiers and analysed at ICES.
Supplemental material
Study eligibility required that an individual was aged 12+ years, a resident of Ontario, had undergone ABO testing and also did not have an SARS-CoV-2 positive swab before 15 January 2021 (online supplemental table S1 and figure S1).
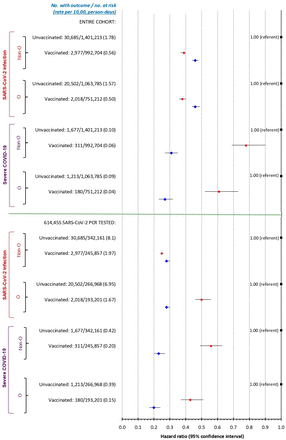
SARS-CoV-2 vaccination and associated risk of SARS-CoV-2 infection, or severe COVID-19 (hospitalisation or death), stratified by O and non-O blood groups. Data are presented for the entire cohort (upper panel), and 614 455 individuals who had SARS-CoV-2 PCR testing during the follow-up period (lower panel). Analyses are by time-varying exposure after first vaccination. Unadjusted HRs are in red, and adjusted HRs in blue, adjusted for age, sex, rural residence, area income quintile—each at baseline—as well as prior diabetes mellitus, malignancy, heart failure, cardiac ischaemia or arrhythmia, chronic kidney disease or venous thromboembolism.
Exposures and outcomes
The main study exposure was a first SARS-CoV-2 vaccination, handled in a time-varying manner, with a lag of 14 days after vaccination to ensure that the person had a chance to develop immunity. High-risk persons were first vaccinated on 15 December 2020, and Canada started mass vaccinating its citizens on 15 January 2021.11 So, for example, a person who was vaccinated on 1 January 2021 or earlier was considered exposed on 15 January 2021, whereas a person who was vaccinated on 1 February 2021 was considered exposed on 15 February 2021 and unexposed before that date.
The main study outcome was SARS-CoV-2 infection, defined as a positive SARS-CoV-2 PCR test—regardless of indication, symptoms or illness severity—arising during the follow-up period, from 15 January 2021 to 27 June 2021. The second study outcome was a severe COVID-19, defined as a positive SARS-CoV-2 PCR test in conjunction with either a hospitalisation within ±3 days, or a death within −1 to +3 days, of that positive PCR test. A ±3-day margin was to allow for PCR testing antecedent to, or following, the hospital admission. The −1 day window permitted the possibility that a PCR specimen was labelled on the day following a COVID-19 death. Both study outcomes were assessed starting at least 14 days after vaccination, among those vaccinated12 (online supplemental table S1).
Data analyses
For the overall cohort, study outcomes were based on population-at-risk denominators, which included both those who did and did not necessarily undergo SARS-CoV-2 PCR testing after 15 January 2021. Time-to-event analyses generated incidence rates, and Cox proportional hazard models produced unadjusted and adjusted HRs, comparing first-vaccinated to unvaccinated persons (referent). Censoring occurred if a person lost their Ontario Health Insurance Plan coverage, were outcome-free by 27 June 2021 (the end of study period), or the day after they died (if a death occurred). HRs were adjusted for age, sex, rural residence, area income quintile—each at baseline—as well as prior diabetes mellitus, malignancy, heart failure, cardiac ischaemia or arrhythmia, chronic kidney disease or venous thromboembolism (online supplemental table S1). Additional analysis 1, (online supplemental table S2) restricted the at-risk denominator for those individuals who underwent SARS-CoV-2 PCR testing at least 14 days after their first vaccination.
The main cohort model was repeated, with each study outcome assessed by more specifically comparing first mRNA vaccination or first Ad-V vaccination to unvaccinated persons (referent).
Next, and central to the study, we examined the risk of each study outcome in relation to first-vaccination status, further stratified by O and non-O blood groups. This was done among the entire cohort, as well as restricted to those who underwent SARS-CoV-2 PCR testing at least 14 days after their first vaccination.
Consideration was given to receipt of a second vaccination as a time-dependent variable. Hence, ‘fully vaccinated’ and ‘partially vaccinated’ persons were each compared with unvaccinated individuals, stratified by O and non-O groups—with these analyses conducted among the whole cohort, as well as limited to just those who had SARS-CoV-2 testing in the observation period.
Analyses were planned a priori. Statistical analyses were performed using SAS V.9.4 for UNIX (SAS Institute, Cary, North Carolina, USA).
Patient and public involvement
No patient was consulted or involved in this study.
Did we involve patients/service users/carers/lay people in the design of this study? No.
Was the development of outcome measures informed by patients’ priorities, experience and preferences? No.
Were patients/carers/lay people involved in the recruitment to and conduct of the study? No.
How will the results be disseminated to study participants? Not applicable.
Are patients/carers/lay people thanked in the contributorship statement/acknowledgements? Not applicable.
Was the development of the research question and outcome measures informed by patients’ priorities, experience and preferences? No.
Results
Among 2 938 215 individuals, 2 472 261 met the inclusion criteria (online supplemental figure S1). Of these, 1 743 916 (70.5%) had at least one vaccination (table 1). Those vaccinated were more likely to be women, older in age, residing in a higher-income area and have higher rates of certain comorbid conditions, like cancer, diabetes and hypertension (table 1). Of those vaccinated, 1 600 524 (91.8%) first received an mRNA vaccine, and 143 358 (8.2%) an Ad-V vaccine. A second vaccine was administered to 24.6% of individuals by 13 June 2021 (ie, by 2 weeks before the end of the study observation period), comprising the mRNA vaccine among 415 632 (23.8%) and the Ad-V among 12 855 (0.7%) (table 1).
Characteristics of 2 472 261 individuals in Ontario, Canada, aged 12 years and older, with known ABO blood group and without evidence of SARS-CoV-2 infection before 15 January 2021. All data are presented as a number (%) unless otherwise indicated
After a median follow-up of 163 days (IQR 163–163), the rate of SARS-CoV-2 positivity was 0.54 per 10 000 person-days among first-vaccinated persons, and 1.69 per 10 000 person-days among non-vaccinated persons—an unadjusted HR of 0.38 (95% CI 0.37 to 0.39) and an adjusted HR of 0.46 (95% CI 0.45 to 0.48). The corresponding HR were equally protective for those receiving a first mRNA vaccine (adjusted HR 0.46, 95% CI 0.44 to 0.47) or first Ad-V vaccine (adjusted HR 0.49, 95% CI 0.44 to 0.54), each relative to being unvaccinated (table 2). The adjusted HR for severe COVID-19 was 0.29 (95% CI 0.26 to 0.33) comparing vaccinated to unvaccinated persons, with similar estimates by vaccine type (table 2).
SARS-CoV-2 vaccination and associated risk of SARS-CoV-2 infection, or severe COVID-19 (hospitalisation or death)—each assessed starting at least 14 days after the first vaccination, among the entire cohort. Data are presented by time-varying exposure after first vaccination versus unvaccinated (upper blue), as well as by first-vaccination type versus unvaccinated (lower maroon)
There were 439 058 (25.2%) vaccinated people who had SARS-CoV-2 PCR testing during follow-up period, from 15 January 2021 onward, compared with 175 397 (24.1%) unvaccinated individuals—a small standardised difference of 0.03. Restricting the at-risk denominator to 614 455 individuals, and comparing the vaccinated to the unvaccinated, the adjusted HR were 0.28 (95% CI 0.27 to 0.29) for SARS-CoV-2 positivity, and 0.22 (95% CI 0.20 to 0.25) for severe COVID-19, although, at much higher event rates than seen in the entire cohort (Additional analysis 1, online supplemental table S2).
Among the entire cohort, the protective effect associated with a first mRNA or Ad-V vaccine against SARS-CoV-2 infection or severe COVID-19 was equally seen among those with O and non-O blood groups (figure 1, upper). This pattern was also seen by vaccine type (online supplemental table S3), and among the 614 455 individuals who had SARS-CoV-2 PCR testing (figure 1, lower).
In the entire cohort, relative to the unvaccinated, fully vaccinated individuals had the lowest risk of SARS-CoV-2 infection, followed by partially vaccinated persons (figure 2, upper). For example, among those with blood group O, the corresponding adjusted HR were 0.39 (95% CI 0.34 to 0.43) and 0.48 (95% CI 0.45 to 0.50). Moreover, the HRs did not differ by blood group. The same was evident for severe COVID-19 (figure 2, upper). Restricting to the subcohort who had SARS-CoV-2 testing, the protective effect conferred by full and partial vaccination was similar by blood groups (figure 2, lower).
Full or partial SARS-CoV-2 vaccination and associated risk of SARS-CoV-2 infection, or severe COVID-19 (hospitalisation or death), stratified by O and non-O blood groups. Data are presented for the entire cohort (upper panel), and 614 455 individuals who had SARS-CoV-2 PCR testing during the follow-up period (lower panel). Analyses are by time-varying exposure after first vaccination. Unadjusted HRs are in red, and adjusted HRs in blue, adjusted for age, sex, rural residence, area income quintile—each at baseline—as well as prior diabetes mellitus, malignancy, heart failure, cardiac ischaemia or arrhythmia, chronic kidney disease or venous thromboembolism.
Discussion
Main findings
This population-based cohort study observed a lower risk of SARS-CoV-2 infection, as well as severe COVID-19 hospitalisation or death, in association with SARS-CoV-2 vaccination. This conferred protective effect did not vary by vaccine type or blood group, but was more pronounced among fully, than partially, vaccinated individuals.
Comparison with other studies
A 2021 meta-analysis of 54 218 persons showed a lower risk of SARS-CoV-2 infection comparing O versus non-O blood group (OR 0.71, 95% CI 0.60 to 0.84).13 In a cohort study of 225 556 adults and children in Ontario, before SARS-CoV-2 vaccination, we previously observed a lower-relative risk of SARS-CoV-2 infection (0.88, 95% CI 0.84 to 0.92) and severe COVID-19 illness or death (0.87, 95% CI 0.78 to 0.97) among those with O versus non-O blood group.6 The current study is the first to explore effect modification of O blood group on vaccine effectiveness against SARS-CoV-2 infection or related illness. Just as we found no effect modification, prior research on the smallpox vaccine suggested no differences in ‘vaccine success’ by ABO blood group,14 nor for influenza A,15 rabies16 and cholera17 vaccines. Thus, if O blood group is somehow protective against SARS-CoV-2 infection or illness, it is unlikely to generate any additive benefit to that conferred by available mRNA and Ad-V vaccines.
The current study observed a relative risk reduction against severe COVID-19 of between 82% and 85% after full vaccination, and between 67% and 70% following partial vaccination (figure 2). In Chile, among those aged 60+ years and fully vaccinated, vaccine effectiveness was 67% against infection, 85% for the prevention of hospitalisation and 87% for the prevention of COVID-19-related death, with corresponding estimates of 16%, 37% and 46% after partial vaccination.18 Our findings about vaccine effectiveness are similar to those of randomised clinical trials of SARS-CoV-2 vaccination,1–4 or another observational study from Ontario.10 Taken together, SARS-CoV-2 vaccination by mRNA or Ad-V is effective at preventing serious disease.
Limitations
This study was limited to persons who had ABO blood group testing, and who are more likely to have required blood transfusion or to have been pregnant in the past.6 As a study strength, identification of blood group status preceded SARS-CoV-2 vaccination or index PCR testing. While we excluded those with SARS-CoV-2 infection prior to 15 January 2021, we did not know who had acquired natural immunity. While vaccination and study outcomes were fully ascertained within a universal healthcare system, a minority of individuals may have been vaccinated outside of Ontario and not identified herein. The potential for immortal time bias—the influence of misclassified follow-up time for individuals who were vaccinated, which could differentially favour their survival—was mitigated by treating vaccine exposure as time varying and by setting follow-up time to a common starting date of 15 January 2021.19 All study covariates, including demographic and clinical variables, were captured prior to time zero. A protective effect of vaccination was seen in the additional analyses restricted to those who underwent PCR testing. This was akin to using a test-negative design, in which common access to, and uptake of, medical care can reduce unmeasured confounding related to healthcare-seeking behaviours.20 While the current study was largely completed prior to the emergence of the SARS-CoV-2 Delta/B.1.617 variant, it is unlikely that ABO blood group would be expected to modify vaccine effectiveness within the subsequent period. Last, adverse events following immunisation were not studied herein, nor the tendency for such adverse events related to ABO blood group.
Conclusions
The protective benefit offered by mRNA or Ad-V SARS-CoV-2 vaccination—especially full vaccination—is not further modulated by ABO blood group status. Large-scale population or targeted vaccination programmes should continue, with ongoing research about how to mitigate emerging viral variants.
Data availability statement
No data are available. No additional data available.
Ethics statements
Patient consent for publication
Ethics approval
The use of data in this project was authorised under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JR and ALP: Study concept, analysis and interpretation of the data, drafting of manuscript, manuscript revision and approval of final version.
Funding Funded by a grant from the Ontario Academic Health Sciences Centre AFP Innovation Fund, and the Ontario Ministry of Health (MOH).This study was also supported by ICES, which is funded by an annual grant from the Ontario MOH and the Ministry of Long-Term Care. Parts of this material are based on data and information compiled and provided by MOH and the Canadian Institute for Health Information.
Disclaimer The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.