Article Text
Abstract
Objectives To investigate the independent and collective impact of alcohol drinking and tobacco smoking on the drug-resistance of newly diagnosed tuberculosis (TB).
Design This was a retrospective cohort study.
Setting Shandong, China.
Participants Patients with newly diagnosed TB from 1 January 2004 to 31 December 2020 were collected. Exclusive criteria: retreated cases; extrapulmonary tuberculosis; without information on drug susceptibility testing results, smoking or drinking habits; bacteriological identification as non-tuberculous mycobacteria.
Primary and secondary outcome measures Patients were classified into four groups including smokers only (G1), drinker only (G2), smoker +drinker (G3), non-smoker +non-drinker group (G0). We described the drug-resistant profiles, clinical factors and calculated the ORs of different drug-resistance among G1, G2, G3, compared with G0 through univariate and multivariate logistics regression models.
Results Of the 7996 TB cases enrolled, the proportions of G1, G2, G3 and G0 were 8.25%, 3.89%, 16.46% and 71.40%, respectively. The rates of drug-resistant (DR)-TB, mono-resistant TB, multidrug resistant (MDR)-TB, polydrug resistant TB in G1, G2, G3 and G0 were 19.24%/16.4%/17.33%/19.08%, 11.52%/8.68%/10.94%/11.63%, 3.03%/2.57%/2.96%/3.66% and 4.70%/4.82%/3.34%/ 4.08%, respectively. G3 had a higher risk of MDR1: isoniazid +rifampin (adjusted OR (aOR)=1.91, 95% CI: 1.036 to 3.532), but had a lower risk of DR-TB (aOR=0.84, 95% CI: 0.71 to 0.99), rifampin-related resistance (aOR=0.68, 95% CI: 0.49 to 0.93), streptomycin-related resistance (aOR=0.82, 95% CI: 0.68 to 0.99), ethambutol-related resistance (aOR=0.57, 95% CI: 0.34 to 0.95), MDR3: isoniazid +rifampin+streptomycin (aOR=0.41, 95% CI: 0.19 to 0.85), any isoniazid +streptomycin resistance (aOR=0.85, 95% CI: 0.71 to 1.00). However, there were no significant differences between G1 and G0, G2 and G0 in all drug-resistant subtypes. Those patients with cavity had a higher risk of DR-TB among G3 (OR=1.35, 95% CI: 1.01 to 1.81).
Conclusion Although we did not found an independent impact of alcohol drinking or tobacco smoking on TB drug-resistance, respectively, these two habits had a combined effect on TB drug-resistance.
- Tuberculosis
- Respiratory infections
- Risk management
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Our study explored the combined impact and investigated the independent effects of smoking and drinking on tuberculosis (TB) resistance.
The data had an excellent scale and period, and were collected in Shandong, China, from 2004 to 2020.
A disadvantage was that smoking and drinking status were not divided into more subgroups because its a retrospective model.
Another limitation was that drug susceptibility testing of second-line anti-TB drugs was not routinely conducted in China.
Introduction
Tuberculosis (TB) has become one of the top 10 causes of death for many years, and it has led to a global threat to health security, while the development of drug resistance among TB even makes this situation worse.1 To achieve the global TB control targets, more attention should be paid to drug-resistant TB (DR-TB), especially multidrug resistant (MDR)-TB, defined as phenotypic resistance to at least isoniazid and rifampin.1 According to the WHO Tuberculosis report, among the estimated 10 million new cases of active TB in 2019, 8.4% were from China (only behind India and Indonesia) and 3.3% were MDR-TB/rifampin-resistant TB (RR-TB).1 TB infection has caused 1.4 million deaths in 2019. The overall success rate of TB treatment was 83%, whereas it was only 54% for MDR-TB/RR-TB and 30% for extensively drug-resistant TB (XDR-TB).2 In recent years, plenty of factors including incomplete treatment, retreated TB, men, TB contact history, HIV and diabetes have been related to the emergence of DR-TB.3 4 Nevertheless, determining more clinical predictors of DR-TB will be conducive to the early detection of DR-TB, and it can also guide the empirical selection of anti-TB drugs, especially in low-income areas where were unavailable to drug susceptibility testing (DST).4
Both tobacco smoking and alcohol drinking were major public health problems.5 6 There were approximately 1.3 billion tobacco users globally, and more than 80% of these populations lived in low-income and middle-income countries.5 The annual global average alcohol consumption among people older than 15 years of age worldwide increased from 5.5 litres of pure alcohol in 2005 to 6.4 litres in 2016.6 Globally tobacco and alcohol consumption causes 8 and 2.8 million premature deaths per year, respectively.5 6 According to two cross-sectional surveys in Shandong province, the rates of drinking and smoking were as high as 64.6% and 46.5% among men, 36.9% and 23.7% among overall residents (more than 18 years old), respectively.7 8 It is estimated that over 20% of adult TB cases may be attributable to smoking, compared with 16% for HIV and 15% for diabetes. In comparison, about 10% of the TB cases globally were estimated to be attributable to alcohol.9 According to previous reports, tobacco smoking doubles the risk of TB infection, and leads to a twice risk of death during TB therapy.10 In addition, alcohol drinking contributed to a 35% higher risk of TB infection (relative risk=1.35, 95% CI: 1.09 to 1.68), accompanied by a lower rate of sputum culture conversion and a worse treatment outcome.11 12In current years, some studies found that smoking (adjusted OR (aOR)=4, 95% CI: 1.2 to 13.2) and alcohol drinking habits (aOR=5.1, 95% CI: 1.4 to 18.7) might be independent predictors for MDR-TB.13 However, most of these studies had a small sample size, more confounding factors such as retreated TB cases and few discussed the combined impact of alcohol uses and tobacco smoking on DR-TB.4 11–13 It also remains unclear the impact of tobacco smoking and alcohol drinking on various drug-resistant subtypes such as mono-resistant (MR)-TB, MDR-TB and polydrug resistant (PDR)-TB.
In this study, based on the retrospective data of Shandong, China, from 2004 to 2020, we aimed to investigate the impact of alcohol drinking and tobacco smoking on the drug-resistance of newly diagnosed tuberculosis in the following aspects: First, we described the clinical features and the drug-resistant profiles of smokers only (G1), drinker only (G2), smoker +drinker (G3) and non-smoker +non-drinker group (G0), respectively. Second, the ORs of different drug-resistance among G1, G2, G3 compared with G0 were calculated. Finally, we also explored the risk factors of DR-TB among G3 and G0.
Participants and methods
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Study design and settings
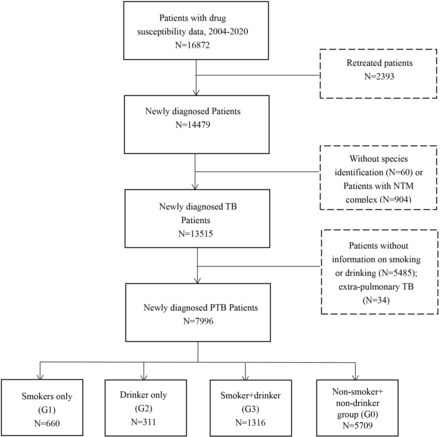
This was a retrospective study of baseline data including DST results, smoking or drinking behaviour among newly diagnosed pulmonary TB cases collected from 30 Shandong, China, surveillance sites (Decheng district, Linqing city, Shen county, Dongming County, Shan county, Yuncheng county, Yanzhou city, Zoucheng city, Sishui county, Xintai city, Changqing district, Licheng district, Jiyang county, Zouping county, Zhouchun district, Gaoqing county, Linqu county, Changle county, Yishui county, Fei county, Cangshan county, Tancheng county, Linshu county, Jiaonan city, Laizhou city, Longkou city, Penglai city, Zhiguan district, Laiyang city, Rushan city) between 1 January 2004 and 31 December 2020. Exclusion criteria: retreated cases; extrapulmonary tuberculosis; without information on DST results, smoking or drinking habits; bacteriological identification as non-tuberculous mycobacteria. Finally, 7996 eligible participants were enrolled (figure 1).
Flowchart of the patient inclusion process. NTM, non-tuberculous mycobacterium; PTB, pulmonary tuberculosis; TB, tuberculosis.
Shandong is the second most populous province in China, with 100.7 million population in 2019. Although the incidence rate of pulmonary TB in Shandong reduced from 40.8 to 26.25 per 100 000 from 2005 to 2017, the aggravation of bacterial drug resistance makes the epidemic situation of TB still severe.1 14 The rates of DR-TB, MR-TB, MDR-TB and PDR-TB in Shandong were 21.38%, 13.35%, 3.73% and 4.30% in 2018, respectively.15
Bacterial culture, strain identification and DST
At least two sputum or bronchoscopy fluid samples were obtained from each suspected TB case. Smear microscopy was performed, and then cultures were conducted in solid Lowenstein-Jensen medium. All cultures with growing colonies were sent to the Reference Laboratory of Katharine Hsu International Research Center of Human Infectious Diseases in Shandong Provincial Chest Hospital. Laboratory technicians identified the strain types by morphology and growth characters of colony, and inhibition of p-nitrobenzoic acid. DST of four first-line anti-TB drugs was routinely performed, including isoniazid (INH, 0.2 µg/mL), rifampicin (RIF, 40 µg/mL), ethambutol (EMB, 2 µg/mL) and streptomycin (SM, 4 µg/mL). If the growth rate was higher than 1% compared with the standard reference strain H37Rv, this strain was defined as resistant to the corresponding anti-TB drug. Superior Reference Laboratory assessed quality control.
Definitions
Definition of smokers and drinkers: (1) Smokers were defined as subjects who had smoked at least 100 cigarettes in their lifetime and smoked at least once in the past 30 days, and vice versa for non-smokers; (2) Drinkers for alcohol drinking refer to those who consumed any alcohol drinking such as beer, liqueur, brandy, whiskey 12 times and at least one alcohol drinking in the past year, and vice versa for non-drinkers.
TB drug resistance types16: (1) MR-TB is defined as resistance to one first-line anti-TB drugs in vitro; (2) MDR-TB is defined as TB infection showing resistance to at least both INH and RIF in vitro; (3) PDR-TB refers to TB with resistance to at least two first-line anti-TB drugs, other than both INH and RIF in vitro; (4) DR-TB refers to TB with any resistance to anti-TB drugs in vitro.
Data analysis
The demographic and clinical characteristics of smokers only (G1), drinker only (G2), smoker +drinker (G3) were compared with non-smoker +non-drinker group (G0) through χ2 tests or Fisher’s exact test also described drug-resistant profiles of these four groups. In addition, we divided the drug-resistance of TB into many subtypes including DR-TB, MR-TB, MDR-TB, PDR-TB, INH-related resistance, RIF-related resistance, SM-related resistance, EMB-related resistance, MDR1: INH+RIF, MDR2: INH+RIF+EMB+SM, MDR3: INH+RIF+ SM, PDR2: INH+SM, PDR3: RIF+SM, any INH+SM resistance, and then multivariate and univariate logistic regression models were applied to estimate the OR, aOR and its 95% CI of different drug-resistant subtype among G1, G2, G3 compared with G0. According to previous studies, covariates such as age, sex, cavity, body mass index, comorbidity were included in models. Finally, we calculated the risk factors of DR-TB among G3. All statistical tests were two-sided with a significance level of 0.05. All above analysis were performed in SPSS statistical software (V.20.0, SPSS, Chicago, USA).
Results
Patients’ characteristics
A total of 7996 newly diagnosed tuberculosis cases were enrolled (table 1), of which 8.25% (660) were smokers only (G1), 3.89% (311) were drinker only (G2), 16.46% (1316) were smoker +drinker (G3) and 71.40% (5709) were non-smoker +non-drinker group (G0). Compared with G0, G1 and G3 were less likely to be aged between 15 and 24 (the corresponding proportions of G1, G3, G0: 7.58%/5.88%/16.75%), 25 and 44 (the corresponding proportions of G1, G3, G0: 19.85%/20.00%/26.83%), but G1 and G3 were more likely to be aged between 45 and 64 (the corresponding proportions of G1, G3, G0: 35.76%/47.56%/29.69%). G1 had a higher proportion but G2 had a lower proportion of the 65+ group than G0 (G1/G2/G0: 35.76%/20.39%/26.32%). G1, G2, G3 were more likely to be men (G1/G2/G3/G0: 99.09%/99.68%/99.7%/77.54%), had comorbidities (total) (G1/G2/G3/G0: 18.94%/17.04%/16.87%/11.68%) or diabetes (G1/G2/G3/G0: 8.94/8.36%/10.03%/6.60%) than G0. G1 and G3 had a higher rate of hypertension than G0, but G2 had a lower rate (G1/G2/G3/G0: 3.33%/0.96%/3.27%/2.05%). Interestingly, we found G1 were more likely to have a chronic obstructive pulmonary disease (G1 vs G0: 3.79% vs 1.87%) and hepatitis (G1 vs G0: 1.82% vs 0.68%). Moreover, the percentage of cavities in G2 was lower but higher in G3 (G2/G3/G0: 38.25%/47.86%/44.43%). G2 was more likely to be overweight than G0 (7.95% vs 5.21%). All of the above were of statistical significance, p<0.05.
Characteristics of 7996 newly-diagnosed pulmonary tuberculosis
Drug resistance patterns
As presented in table 2, the proportions of DR-TB, MR-TB, MDR-TB, PDR-TB in G1, G2, G3 and G0 were 19.24%/16.4%/17.33%/19.08%, 11.52%/8.68%/10.94%/ 11.63%, 3.03%/2.57%/2.96%/3.66% and 4.70%/4.82%/3.34%/4.08%, respectively. Compared with G0, G3 had more MDR1: INH+RIF (1.29% vs 0.65%) but less RIF-related resistance (3.80% vs 5.20%) and MDR3: INH+RIF+ SM (0.61% vs 1.56%).
Drug resistant profiles among TB cases with or without alcohol drinking and tobacco smoking habits
The impact of alcohol drinking and tobacco smoking on TB drug-resistance
Smoker +drinker (G3) had a higher risk of MDR1: INH+RIF (OR=2.01, 95% CI: 1.13 to 3.57, p=0.018; aOR=1.91, 95% CI: 1.04 to 3.53, p=0.038), but had a lower risk of DR-TB (aOR=0.84, 95% CI: 0.71 to 0.99, p=0.035), RIF-related resistance (OR=0.72, 95% CI:0.53 to 0.98, p=0.035; aOR=0.68, 95% CI: 0.49 to 0.93, p=0.015), SM-related resistance (aOR=0.82, 95% CI: 0.68 to 0.99, p=0.042), EMB-related resistance (aOR=0.57, 95% CI: 0.34 to 0.95, p=0.032), MDR3: INH+RIF+ SM (OR=0.39, 95% CI: 0.19 to 0.80, p=0.01; aOR=0.406, 95% CI: 0.19 to 0.85, p=0.017), any INH+SM resistance (aOR=0.85, 95% CI: 0.71 to 1.00, p=0.05). However, there were no significant differences between G1 and G0, G2 and G0 in all drug-resistant subtypes (table 3).
Association between alcohol drinking, tobacco smoking and TB drug resistance
Risk factors for DR-TB
As shown in table 4 and table 5, (1) TB cases with alcohol drinking and tobacco smoking habits: Those who with cavity had a higher risk of DR-TB (OR=1.35, 95% CI: 1.01 to 1.81, p=0.042); (2) TB cases without alcohol drinking and tobacco smoking habits: Men (OR=1.24, 95% CI: 1.05 to 1.46, p=0.011; aOR=1.26, 95% CI: 1.08 to 1.49, p=0.007), cavitary disease (OR=1.17, 95% CI:1.02 to 1.35, p=0.03) might increase the risk of DR-TB among non-smoker +non-drinker group (G0). On the contrary, TB cases aged more than 65 were less likely to have DR-TB (OR=0.78, 95% CI: 0.63 to 0.97, p=0.023).
Univariable and multivariable analysis of risk factors for DR-TB in smoker +drinker
Univariable and multivariable analysis of risk factors for DR-TB in non-smoker +non-drinker
Discussion
Based on 7996 newly diagnosed tuberculosis cases in Shandong, China, we sought to investigate the independent and combined effect of alcohol drinking and tobacco smoking on TB drug-resistance, respectively. In short, smoker +drinker (G3) had a much higher risk of MDR1 (INH+RIF) but they had a lower risk of MDR3 (INH+RIF+ SM) and any INH+SM resistance. When divided into any first-line anti-TB resistance, we found that G3 were less likely to be RIF-related resistance, SM-related resistance, EMB-related resistance. Interestingly, when analysed at the overall level of TB resistance, we found that G3 were less likely to be DR-TB compared with G0. However, there was no significant impact of alcohol drinking only or tobacco smoking only on various drug-resistant subtypes (p>0.05). Compared with the control (G0), whether TB cases belonged to smokers only(G1), drinker only (G2) or G3, they all had more men, comorbidities (total) and diabetes. Furthermore, men and cavitary diseases were more likely to be DR-TB among the non-smoker +non-drinker group (G0).
According to our study, compared with G0, TB cases with both drinking and smoking habits had different drug-resistant profiles such as an increased risk of MDR1 (INH+RIF) but a lower risk of DR-TB. Our findings were not identical to a previous meta-analysis which showed that smoking habits were associated with an increased risk of DR-TB (OR=1.57, 95% CI: 1.33 to 1.86), MDR-TB (OR=1.49, 95% CI: 1.19 to 1.86).17 Another study found that alcohol abuse (OR=1.3; 95% CI: 1.0 to 1.8) was a risk factor identified for MDR-TB.18 However, the absence of an correlation between alcohol drinking only, tobacco smoking only with TB resistance in our research was inconsistent with the findings of most previous studies.17 18
As we all know, many factors such as incomplete and inadequate treatment, complications of diabetes, direct transmission of drug-resistant strains contribute to the development of DR-TB.4 18 19 Therefore, alcohol drinking and tobacco smoking may also affect the resistance of TB through the above pathways. There may be some explanations for the combined impact of both drinking and smoking habits on TB resistance: (1) Tobacco smoking and TB resistance: It has been found that tobacco smoking was associated with the evaluated risk of TB infection, the increased TB-related mortality and the lower treatment compliance.9 10 The roles of tobacco smoking in the pathogenesis of TB were that cigarette smoking decreased the mucociliary clearance, reduced the immune response of alveolar macrophage, led to lower production of tumour necrosis factor-α and interleukin-12, and impeded granuloma formation, thus creating conditions for the infection and development of TB.10 20 Previous studies suggested that mutations associated with TB resistance usually lead to an impaired bacterial growth rate and decreased virulence known as ‘fitness cost’.21 Presumably, reduced immune function in the human body caused by tobacco smoking may contribute to the infection of some drug-resistant TB strains such as MDR1. In addition, TB cases with tobacco smoking had lower compliance and were less likely to complete anti-TB therapy, resulting in acquired resistance.17 22 (2) Alcohol drinking and TB resistance: Similar to smokers, TB cases with alcohol drinking habits also had poorer treatment outcomes including loss to follow-up, death, treatment failure among both sensitive TB (OR=1.99, 95% CI: 1.57 to 2.51) and MDR-TB (OR=2.00, 95% CI: 1.73 to 2.32), while treatment failure was a independent risk factor for acquired resistance.23 24 Alcohol use could also lead to weakened immunity, liver damage and nutritional deficiency, contributing to sensitive and resistant TB infection.25 Interestingly, we found a protective effect of alcohol drinking and tobacco smoking on resistance. The altered susceptibility may also cause it to different TB strains, but more potential mechanisms remain to be explored. Finally, our study indicated that the combined effects of alcohol drinking and tobacco smoking might be more substantial than alone, which may explain the results without statistical significance among drinker-only and smoker-only groups.
People with both smoking and drinking habits were more likely to be men (accounted for 99.7%) and aged between 45 and 64 years (accounted for 47.56%). They also had higher comorbidities (16.87%) and diabetes (10.03%). The gender difference in smoking and drinking habits is huge. For example, a population-based study in China found that 45% of men and 3% of women were cigarette smokers and 34% of men and 4% of women were alcohol drinkers.26 TB cases also have more men, and it was reported that about 6 million adult men and 3.2 million adult women fell ill with TB in 2017.27 Smoking and drinking have many adverse effects on human health, and they could increase the risk of cardiovascular disease, stroke, infection, diabetes and so on.11 22 28
Among TB cases with drinking and smoking habits, those with cavities had a higher risk of DR-TB, while among TB cases without these habits, both men and cavitary disease were risk factors for DR-TB. So far, studies on the association of gender and TB resistance were still not consistent, some found women were more likely to have MDR-TB than men (aOR=1.315 95% CI: 1.117 to 1.548, p=0.001), but another found that either sex was at higher risk of MDR/RR-TB.29 30 Different gender often means differences in living habits such as smoking and drinking, social pressures, access to healthcare services and exposure to other risk factors.30 31 Both men and TB diseases with cavities should be recognised as a vulnerable population to DR-TB, and improve their DST access. A directly-observed treatment strategy may help reduce the burden of TB in China more effectively.
Our study has several strengths. First, although the combined effects of smoking and drinking on TB resistance, including many subtypes, were rarely discussed in former publications, our study explored the combined impact and investigated the independent effects. Second, our study had an excellent scale and period, and we collected all newly diagnosed TB cases with DST results, smoking and drinking status in Shandong, China, from 2004 to 2020. Third, our study was conducted among new TB cases after excluding all retreated cases, which would help to reduce more confounding factors. A disadvantage of our study was that it had not divided smoking and drinking status into more subgroups. In addition, we only used the available information because it was a retrospective study. Another limitation was that DST of second-line anti-TB drugs was not routinely conducted in China unless the patient asked for it.
Conclusion
Although we did not find an independent impact of alcohol drinking or tobacco smoking on TB drug-resistance, respectively, these two habits had a combined effect on TB drug-resistance. Smoker +drinker (G3) had a higher risk of MDR1 (INH+RIF), but had a lower risk of DR-TB, RIF-related resistance, SM-related resistance, EMB-related resistance, MDR3 (INH+RIF+ SM) and any INH+SM resistance. TB cases which belonged to smokers only (G1), drinker only (G2) or G3, were more likely to be men, or combined with comorbidities (total) and diabetes, meanwhile cavitary disease were risk factors for DR-TB among G3. In short, considering the combined impact of alcohol drinking or tobacco smoking on TB drug-resistance, we should be alert for the emergence of INH+RIF resistance among these populations.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The Ethics Committee approved the protocols applied in this study of Shandong Provincial Hospital, affiliated with Shandong University (SPH), and the Ethics Committee of Shandong Provincial Chest Hospital (SPCH) China. Personal identifiers of patients with tuberculosis were removed before data analysis and reporting. Relevant guidelines and regulations performed all methods.
Acknowledgments
We thank Shandong Provincial Hospital, Shandong Provincial Chest Hospital, 13 municipal-level and 21 county-level local health departments for drug susceptibility data, sociodemographic and clinical data.
References
Footnotes
W-mS, S-jL and J-yL contributed equally.
Contributors HCL, WMS, SJL, JYL and YFL conceived and designed the study. HCL, CBY, QQA, SQL, QF and JD directed its implementation including the data analysis and writing of the paper. WMS, JD and YFL analysed the data. YL, QYZ, JYL, TTX, SJL and NNT contributed materials/analytical tools. WMS and HCL wrote and revised the manuscript. All authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. HCL is responsible for the overall content and is a guarantor.
Funding This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2020KH013), Department of Science and Technology of Shandong Province (CN) (No.2007GG30002033; No.2017GSF218052) and Jinan Science and Technology Bureau (CN) (No.201704100). The study sponsors had not participated in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.