Article Text
Abstract
Introduction Progress in degenerative cervical myelopathy (DCM) is hindered by inconsistent measurement and reporting. This impedes data aggregation and outcome comparison across studies. This limitation can be reversed by developing a core measurement set (CMS) for DCM research. Previously, the AO Spine Research Objectives and Common Data Elements for DCM (AO Spine RECODE-DCM) defined ‘what’ should be measured in DCM: the next step of this initiative is to determine ‘how’ to measure these features. This protocol outlines the steps necessary for the development of a CMS for DCM research and audit.
Methods and analysis The CMS will be developed in accordance with the guidance developed by the Core Outcome Measures in Effectiveness Trials and the Consensus-based Standards for the selection of health Measurement Instruments. The process involves five phases. In phase 1, the steering committee agreed on the constructs to be measured by sourcing consensus definitions from patients, professionals and the literature. In phases 2 and 3, systematic reviews were conducted to identify tools for each construct and aggregate their evidence. Constructs with and without tools were identified, and scoping reviews were conducted for constructs without tools. Evidence on measurement properties, as well as on timing of assessments, are currently being aggregated. These will be presented in phase 4: a consensus meeting where a multi-disciplinary panel of experts will select the instruments that will form the CMS. Following selection, guidance on the implementation of the CMS will be developed and disseminated (phase 5). A preliminary CMS review scheduled at 4 years from release.
Ethics and dissemination Ethical approval was obtained from the University of Cambridge (HBREC2019.14). Dissemination strategies will include peer-reviewed scientific publications; conference presentations; podcasts; the identification of AO Spine RECODE-DCM ambassadors; and engagement with relevant journals, funders and the DCM community.
- neurosurgery
- neurological injury
- neurological pain
- neuromuscular disease
- adult neurology
- neurology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The core measurement set (CMS) will be established using a robust, global and multi-stakeholder consensus process, with broad representation of healthcare professionals and individuals living with the disease.
The CMS will only focus on measurement instruments currently in use and exclude instruments under development, translational research, or in languages other than English.
Where there are gaps in degenerative cervical myelopathy outcome measurement, systematic and targeted scoping reviews will be performed to identify instruments used in related populations, which are likely but not guaranteed to measure equivalent outcome constructs.
The CMS will be selected using modified nominal group techniques that have been effectively used during previous consensus processes.
Introduction
Background
Degenerative cervical myelopathy (DCM) is a common and often disabling disease.1 Estimated to affect as many as one in fifty adults,1 it develops due to degenerative and/or congenital changes in the cervical spine leading to mechanical stress and a progressive spinal cord injury.2–4 This disease can lead to a wide variety of symptoms, affecting the whole body.5 These symptoms commonly include gait dysfunction, imbalance and falls, loss of strength and manual dexterity, and pain. Despite current best practice,6 a minority of patients will make a full recovery and DCM is often associated with lifelong disability, impaired quality of life and significant costs to both the individual and to society.7 8
While progress has been and is being made,6 9 there remain significant knowledge gaps. For people affected by DCM, solutions to these challenges cannot come soon enough.10 AO Spine Research Objectives and Common Data Elements for Degenerative Cervical Myelopathy (AO Spine RECODE-DCM; www.aospine.org/recode) is an international, multi-stakeholder initiative originally formed to create a ‘research toolkit’ that could help accelerate knowledge discovery and improve outcomes in DCM.11 12 This project aimed to unify terminology, and develop minimum standards for measurement and data reporting,12–14 in order to enable data aggregation and implementation of management recommendations.15–17 The value of addressing these inefficiencies is likely magnified for DCM, as the research community is relatively small, fragmented and has not received commensurate attention or funding.18 19 This is magnified by the use of 14 different names around the world, with common alternatives including cervical spondylotic myelopathy, cervical myelopathy and cervical stenosis.20
So far, AO Spine RECODE-DCM has established the top research priorities and agreed on a single definition and index term.4 8 21–32 It has also agreed on ‘what’ should be measured in DCM research: that is, a minimum data set, which is comprised core data elements (CDE) and a core outcome set (COS). The COS is composed of six domains: neuromuscular function, life impact, pain, radiology, economic impact and adverse events. Each domain contains a list of more specific outcomes that should be measured. While adherence to this minimum dataset should ensure a more comprehensive assessment of DCM, to ensure data is reported in a consistent manner, best suited for between study comparison and evidence synthesis, this standardisation should also extend to ‘how’ the dataset should be measured and reported. This additional phase is referred to as the development of a core measurement set (CMS) (table 1).33–35
Research Objectives and Common Data Elements for DCM definitions and terminology
A CMS is a set of agreed on tools that are used to measure the CDE and COS.36 A CMS is needed to improve the consistency of data measurement and reporting across DCM and will ultimately accelerate changes that will improve outcomes for this population.12 This protocol defines how AO Spine RECODE-DCM will establish a CMS for DCM.
Methods and analysis
Overview and scope
The CMS will continue to be managed within the framework of AO Spine RECODE-DCM.11 Ethical approval for this project was obtained from the University of Cambridge (ethical approval number: HBREC2019.14). A multi-disciplinary, global steering committee (SC) was formed for the oversight of the project (www.aospine.org/recode). In addition to interim correspondence, the committee meets at least two times a year. For a meeting to be considered quorate, it must include at least two people with lived experience and four healthcare professionals. When a steering group member is unable to attend, decisions made at quorate meetings are respected. Day-to-day administration is provided by a multi-stakeholder management group.
As outlined earlier, the standardisation of data measurement and reporting is an immediate priority for DCM. However, the research priority-setting process further recognised a need to develop new measurement instruments for DCM.27 Acknowledging that such development demands a significant period of time and financial support, it was decided that the initial CMS should focus on selecting the most relevant—but existing—instruments, as opposed to developing new tools or selecting those early in development. The added benefit would be to enable comparisons with historic data while simplifying the implementation of DCM’s first minimum dataset. This rationale is expanded in the discussion.
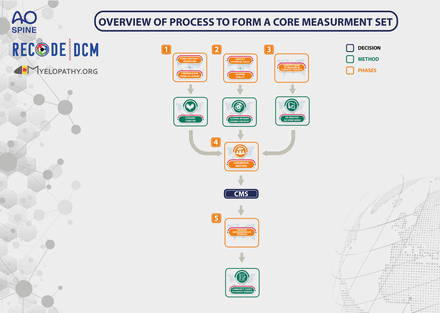
The development of the CMS is based on relevant guidance, including that developed by the Core Outcome Measures in Effectiveness Trials and the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN).36–44 Notably, no more than one measurement tool will be selected per core outcome.36 The developmental process will be conducted in five phases (figure 1):
Phase 1: to agree on the measurement construct and preferred measurement approach.
Phase 2: to identify measurement tools and evaluate their evidence base.
Phase 3: to aggregate the evidence on timing of assessment.
Phase 4: to select the most appropriate instruments through multi-stakeholder consensus and provide reporting guidance.
Phase 5: to implement the CMS
Overview of the core measurement set (CMS) process.
The CMS will cover each element contained within the CDE but each domain of the COS (the minimum dataset). For phases 1 and 2, preparatory scoping work will focus on the specific outcomes but during phase 4 (Consensus), this detail will be used to inform a representative measurement instrument or instruments for the domain as a whole. Elements in the CDE which are descriptive (eg, individual’s age or sex) and do not require measurement per se, will only feature in phases 3 and 4. These elements will be identified and agreed during phase 1.
Information on the status of each phase is shown in table 2. Where a phase has not yet been completed, information on the planned timeline for completion is described as of the time of peer-review.
Status of the CMS process
Gap analysis
Patient and public involvement
This project forms part of a larger, international multi-stakeholder co-production initiative called AO Spine RECODE-DCM, which aims to develop a framework to accelerate knowledge discovery that can improve outcomes in DCM. Patients and the public were therefore involved in its overall design, conduct, management and dissemination, and are recognised among the authors of this article (for further information, refer to www.aospine.org/recode).
Phase 1: forming measurement constructs and establishing the preferred measurement approach
During the formation of the CDE and COS, each element was summarised with a lay description. While this provided an explanation as to how the term was originally proposed, for example, based on content from interviews,5 10 these descriptions were not intended as construct definitions. Further, as some outcomes were merged and/or renamed during the process, they lacked a unifying explanatory statement.
Consequently, the first step of this CMS is to agree on the specific construct to be measured.36–44 These will be expressed by forming a definition for each element. Draft definitions will be generated from original source documents including published literature or interviews with patients and professionals. This will be undertaken by the management group. These provisional definitions will then be reviewed by the SC and iterated as indicated. Each definition must reach >70% approval at a quorate meeting to be considered final.
For elements requiring measurement, the SC will also define through agreement, whether it should be ideally measured by people with DCM (ie, a patient-reported outcome measure, or PROM), a healthcare professional (ie, a clinician-reported outcome measure, or ClinROM), or both. These decisions will not necessarily be considered binding for the final CMS owing to the uncertainty at this stage around the availability and quality of candidate measures. The decision instead will be used during phase 4, to help inform the selection of instruments for the CMS.
Phase 2: identifying potential instruments and evaluating their measurement properties
Phase 2 will be conducted in three stages: (2.1) a systematic review to assess the quality of existing measurement instruments used in DCM; (2.2) a gap analysis of elements, to identify those for which a measurement instrument of sufficient quality within DCM does not exist and (2.3) targeted scoping reviews of these gap elements, to identify potentially relevant instruments used outside of DCM.
Phases 2.1 and 2.2 have been completed. Phase 2.1 has been published separately45; thus, only a summary is provided here. Phase 2.2 and its results are included here.
Systematic review of existing measurement instruments
A systematic review was used to evaluate the quality of a predefined list of existing measurement instruments, identified from three previous scoping reviews.13 45–47 The term ‘measurement instrument’ was used to refer to how the element was being measured (ie, the instrument used to assess the outcome) and could refer to a single question, a questionnaire, or other instruments,48 49 including PROMs and ClinROMs.
The search was performed in EMBASE and MEDLINE from inception until 4 August 2020 to identify original research assessing the measurement properties of instruments used in clinical research of DCM. The search string was built using the relevant DCM search filter50 51 and the COSMIN filter for studies evaluating measurement properties.52 Abstracts were screened by four reviewers against a set of predefined criteria (online supplemental table 1). Only primary clinical research studies evaluating one or more measurement properties were included.
Supplemental material
All data were collected, processed and analysed in accordance with the COSMIN manual for systematic reviews of PROMs. This involved collecting results across 10 measurement properties: content validity, structural validity, internal consistency, cross-cultural validity/measurement invariance, reliability, measurement error, criterion validity, hypotheses testing for construct validity, responsiveness and clinically important differences. Results were rated as ‘sufficient’, ‘indeterminate’ or ‘insufficient’ and overall methodological quality scores were scored as ‘very good’, ‘adequate’, ‘doubtful’, ‘inadequate’ or ‘not applicable’, as described in the manual. Results were then qualitatively summarised and an overall rating of the quality of the studies was made using a modified Grading of Recommendations Assessment, Development and Evaluation approach, as described in the manual. Recommendations were formulated based on all evidence, a list of interpretable instruments was collated and findings were subsequently reported as a narrative synthesis.53
Gap analysis
While the review identified clinically interpretable instruments that were common to DCM research and could be used to measure outcomes in the COS, there were: (a) several elements for which no existing instrument was appropriate and (b) several instruments for which the evidence base was deemed inadequate.36
To identify candidate instruments for these gaps, we looked for appropriate instruments outside of the field of DCM. Before conducting scoping reviews for each gap de novo, a pragmatic MEDLINE search was performed to assert if such reviews already existed. Outcomes within the domain of pain were excluded as it was felt the resources and recommendations aggregated by the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials were sufficient.54 Search strings were formed, comprising the core outcome, synonyms of ‘psychometric’ and ‘Neuroscience’,50 52 and were limited to the last 5 years to ensure relevance. The search was restricted to Neuroscience as it was anticipated this would most likely identify instruments with appropriate content validity. Abstracts were screened by one reviewer against the same criteria from the review (online supplemental table 1). Results from this gap analysis are aggregated in table 3. Notably, no systematic reviews were identified, but a published protocol with respect to fatigue was, and the study results obtained via personal communication.55
Targeted scoping reviews
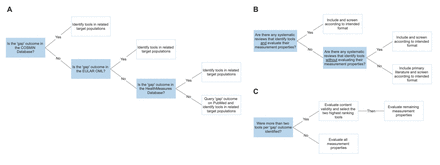
For those remaining outcomes without potential instruments, focused scoping reviews will be conducted. These reviews will be conducted in two stages and will aim to: (a) identify instruments used in a related target population (to increase the likelihood of content validity) and (b) evaluate the methodological quality of those identified instruments. Recognising the intensive undertaking of reviewing the quality of instruments using the COSMIN methodology, in order to ensure this undertaking is manageable and likely to yield relevant results, it will be conducted in the following pragmatic fashion (figure 2):
Stage 1
1.1 Identify tools outside DCM for domains in phase 2.2.
1.2 Screen tools from stage 1.1 according to intended format, that is, ClinROM or PROM.
Decision tree schematic illustrating the targeted scoping review process. (A and B) Stage 1: selection of databases for identification of tools outside degenerative cervical myelopathy (DCM) (A) and screening of tools outside DCM (B). (C) Stage 2: evaluation of measurement properties. COSMIN, Consensus-based Standards for the selection of health Measurement Instruments; EULAR OML, EULAR Outcomes Measures Library.
Stage 2
2.1 Evaluate content validity of PROMs from stage 1.
2.2 Evaluate content validity of ClinROMs from stage 1.
2.3 Select two PROMs and ClinROMs from stages 2.1 and 2.2.
2.4 Evaluate measurement properties of tools selected in stage 2.3.
2.5 Share list of tools with psychometric evaluations ahead of consensus meeting.
To identify instruments, each ‘gap’ outcome will be queried first on the COSMIN database of systematic reviews of outcome measurement instruments (https://database.cosmin.nl/) (figure 2A). As a scoping exercise, each search will focus on reviews in order to develop a list of measurement instruments. Preferably, systematic reviews identifying instruments and evaluating their methodological quality will be included (figure 2B). Where these are not available, systematic reviews identifying instruments without methodological evaluations will be favoured, followed by reviews referred from SC advice and, ultimately, primary literature.
Searches will be conducted in disease populations related to DCM in order to increase the likelihood of content validity. For example, ‘faecal incontinence’, could be a symptom of many diseases. However, since this symptom is also measured in other spinal disorders with neurological injury (eg, traumatic spinal cord injury and cauda-equina syndrome), these disorders would be considered appropriate populations. These will be defined with input from stakeholders a priori.
As in phase 1, instruments will be categorised as PROMs or ClinROMs.56 Only instruments whose category matches the intended outcome category, as defined in phase 1, will be included. Namely, if ‘faecal incontinence’ was defined as a patient-reported outcome during phase 1, then only PROMs of ‘faecal incontinence’ will be included, and ClinROMs will be excluded.
The above steps will be performed for each ‘gap’ outcome in table 3 in order to identify instruments used in related target populations. If no such instruments are found through the COSMIN database, the same steps will be performed on the EULAR Outcomes Measures Library (OML, https://oml.eular.org/) (figure 2A). If no such instruments are found through the EULAR OML, the same search will be performed, as a last resort, on the HealthMeasures Database (https://www.healthmeasures.net/), failing which, the search will be performed on PubMed using the COSMIN filter.52 These databases were selected based on their scope.
To evaluate the methodological quality of the identified instruments, the same COSMIN process as in phase 2.145 will be used. Recognising that evaluating an uncapped number of instruments with the COSMIN manual can quickly become unrealistic, we will limit the number of instruments for COSMIN review to two per ‘gap’ outcome. Should there be more than two PROMs or ClinROMs per ‘gap’ outcome, a content validity survey will be conducted on at least five people with lived experience or clinicians (as applicable) to rank the identified instruments (figure 2C). The two highest ranking instruments will be selected for COSMIN review and their psychometric properties will be evaluated as in phase 2.1.45
Phase 3: evidence on timing of assessment
The timing of the assessment is an additional source of variation with respect to aggregating outcomes. For studies considering non-operative management due to the current uncertainty around the natural history of DCM (recognised as a critical research priority)57 this will not be possible. However, for DCM managed operatively, the recovery profile is more stereotyped and felt amenable to standardisation measurement time points.
To help inform this recommendation, an evaluation of the AO Spine Cervical Spondylotic Myelopathy (CSM) North America and International datasets will be conducted.58 59 These are two high-quality observational studies of patients undergoing surgery for DCM, followed up at 3, 6, 12 and 24 months after surgery. These incorporate the most frequently used follow-up timepoints from DCM research.13 Recovery trajectories will be modelled over time, including the proportion of patients achieving maximal recovery at each follow-up point and the percentage change from last follow-up. The significant of contextual factors that may influence this (eg, age or comorbidities) will also be explored. These findings will be shared during phase 4.
Phase 4: consensus recommendations
Formation of an expert consensus panel
A multi-disciplinary panel of experts will be formed to finalise the CMS through consensus. These experts will be identified using purposive sampling to include people with lived experience; professionals from key clinical disciplines commonly involved in DCM care (ie, spinal surgery, neurology, rehabilitation medicine, physiotherapy and primary care)12 60; professionals with clinical trials experience, particularly with respect to measuring each of the six domains (ie, adverse events, economic impact, life impact, neuromuscular function, pain and radiology); and professionals with experience in trial statistics. A target sample size of 12 individuals will be sought. At least half of all participants will be external to the SC; at least one in six participants will have lived experience; and no more than half of all participants will be spinal surgeons. It is also intended to have a 1:1 ratio of women to men. All panellists must declare any conflicts of interest, and be approved by the SC.
Pre-meeting short-listing
Panellists will be provided with a summary containing the identified measurement instruments considered of sufficient quality for each element, including their evidence base, and the original steering committee decision concerning the preferred reporting method (ie, PROM or ClinROM). Each panellist will be asked to submit two preferred measurement instruments in advance of the meeting. These may include the instruments identified and evaluated during phase 2 or up to two instruments from outside this list. To justify the suggestion of instruments from outside the provided list, panellists will be asked to cite one primary article per psychometric domain (ie, one for validity, one for reliability and one for responsiveness). This literature will be evaluated using the same COSMIN methodology from phases 2.1 and 2.3, to ensure that all instruments presented at the face-to-face consensus meeting are accompanied with a COSMIN rating and comparable.
Face-to-face consensus meeting
A consensus meeting of the panel will then be convened. The aims will be: (a) to select the preferred measurement instruments, (b) to define how they should be reported and (c) to outline when they should be reported in surgically treated DCM cohorts. The management group will prepare documentation for each domain, comprising those instruments shortlisted by the panel during phase 4.2 together with their evidence. Each domain will be discussed in turn with a majority decision considered consensus agreement. Where applicable, this will also continue for each element of the CDE. The consensus meeting will be overseen by an independent facilitator and follow a modified nominal group technique. Moderated discussion and re-voting will be undertaken as necessary until consensus is achieved for all components of the COS and CDE. Consensus will be defined as >70% agreement.
Phase 5: implementation
The dissemination of the CMS will be incorporated into the active knowledge translation proposal for the entire AO Spine RECODE-DCM initiative. This includes scientific publication; conference presentations; podcasts; identifying AO Spine RECODE-DCM ambassadors; and engaging with relevant journals and funders. This process will be subject to periodic review to ensure strategies are effective and adaptive.
This will include a survey of the RECODE-DCM community, designed to share the CMS and ascertain barriers to implementation. This information will be used to inform overall strategy.
The AO Spinal Cord Injury Knowledge Forum, an international and multidisciplinary group of professionals working in this field, will review the relevance of the CMS at 4 years from release, to consider whether an update is required.
Ethics and dissemination
Ethical approval was obtained from the University of Cambridge (HBREC2019.14). Participant consent will be sought for the consensus meeting. Members of the SC have already consented to participate in this study. Dissemination strategies for this project will include scientific publication, presentation and communication, and are described in more detail in phase 5.
Discussion
This protocol outlines the process for developing a CMS for DCM, based on the CDE and COS already defined by AO Spine RECODE-DCM. While some pragmatic steps have been taken, this process remains faithful to consensus methodology and CMS precedent36–44 48 and, ultimately, remains robust.
CMS will focus on measurement instruments currently in usage
From the outset, it was decided that the CMS would principally focus on existing instruments currently in use. Although the development of better assessment instruments is a top 10 research priority,27 the strategy to use existing instruments was preferred for several reasons. First, the aim of this project was to develop a CMS that could be immediately implemented in clinical practice and research studies. The development of new tools remains a work in progress, including microstructural MRI, gait laboratory analysis and clinical assessments.27 30 61 While it seems inevitable that these measurement instruments will change DCM assessment, there remain important methodological uncertainties, practical challenges and technological requirements that pose potential barriers to adoption.
Widespread adoption is necessary for a minimum data set to improve research efficiency. Unless individual DCM researchers have unified data collection, the comparison of findings across studies will remain limited.62 Changing practice, however, is challenging, particularly when a concept is unfamiliar or questioned.63–65 It is therefore important to recognise that CMSs can be updated66 and that individual studies can incorporate additional instruments at their discretion. Furthermore, the inclusion of emerging technology should only be included in future CMS iterations when their selection is undisputable.
For DCM, an equally important but more achievable priority is to ensure that the intended breadth of outcomes is being measured. As highlighted in phase 2.2, previous studies may have underrepresented the disease.13 18 This holds significant implications for interpreting the literature. A recent example is the results of the CSM-Protect study, a randomised controlled trial comparing riluzole as an adjuvant to surgery to surgery alone.67 While there were no differences between treatment groups with respect to the primary endpoint (ie, neuromuscular function), there were indications of meaningful benefit among secondary outcomes (eg, complications such as C5 Nerve Palsy, and pain).
As a nascent research field with a paucity of high-quality prospective studies,6 9 ensuring that current research is comparable to these benchmarks will be important for their generalisation and implementation in the short term.17 This will require existing measurement instruments to be represented.
CMS will be selected using modified nominal group techniques
Several methods exist to achieve meaningful consensus.68 69 Ultimately, these methods aim to ensure that all relevant perspectives are captured and appropriately represented in the decisions taken.70 Consensus processes are increasingly approached by combining literature evidence, serial surveys and a final consensus meeting—a modified Delphi.68 71 72 This approach was effectively used during our previous three consensus processes (ie, for the index term, CDE, and COS).
The diverse perspectives from different stakeholder groups was imperative in determining ‘what’ to measure, identifying previously unprioritised outcomes73 and developing a global multi-stakeholder community focused on DCM.32 Arguably, ‘how’ to measure these outcomes will require further focused perspectives on clinical assessment and trials. When conducting our international Delphi processes, engaging under-represented stakeholders was challenging.12 22 74 At the outset, we aimed to capture perspectives of people with lived experiences, surgeons and other healthcare professionals in a 2:1:1 ratio.12 However, this could not be achieved, and engaging spinal surgeons—who most frequently treat, research, and specialise in DCM—was much easier.22 Given that the CDE and COS have been defined, and that the decision on how to measure them is likely to benefit from specific expertise, a purposively selected group using a modified nominal group technique was favoured for the CMS. It is also hypothesised that the step of sharing the results of the CMS with the wider DCM research community will facilitate dissemination and improve face validity.
Limitations
Despite its conscientious design, this CMS process has limitations. As in Yanez Touzet et al,45 in searching for existing instruments, we have neither identified nor assessed tools under development, or those currently being translated into clinical or research settings, or those published in languages other than English. Further, to ensure that the identification and evaluation of candidate tools in use outside of DCM is manageable, pragmatic steps have been taken. While this risks missing relevant tools, we suspect this is very unlikely to limit the CMS. First, the shortlisting takes a systematic and structured approach, adapted from the prioritisation of databases and standards in the COSMIN website and manual (respectively).37–39 75 This was supplemented by the perspectives of the SC, which includes significant DCM research experience and remains open to suggestions from those attending the consensus meeting.
Notably, in the gap analysis, only one suitable resource was identified out of 973 candidates (table 3). This paucity of high-quality evidence is not surprising given our prior experience with the COSMIN guidelines.45 The COSMIN standards set a high bar for evaluating psychometric assessments. For example, studies on content validity cannot score higher than ‘inadequate’ without focus group/interview recordings or verbatim transcriptions—and, in our experience, most of these studies rely on survey-based methods. These standards have been previously conceived as both strengths, and limitations, of the COSMIN methodology.76–78 That only one outcome out of 28 had one suitable resource was noteworthy at the gap analysis stage but, when interpreted within the context of the psychometric rigour (or stringency) of the guidelines, it is neither surprising nor worrying due to our intent to include the highest possible quality of instruments in this CMS.55
Finally, in resorting to shortlisted instruments used in populations other than DCM, we have introduced the possibility for invalid instruments to be selected. To minimise this limitation, we stipulated that the constructs being measured in these populations must be, in all likelihood, equivalent, that is, there is content validity. This was desirable due to the number of gaps in phase 2.2 and feasible due to the COSMIN recommendations.37–39 As in shortlisting, the option for experts to suggest other instruments prior to the consensus meeting should provide an opportunity to resolve this limitation as much as possible. Alternatively, the expert discussions, voting and re-voting involved in the modified nominal technique should address these concerns explicitly.
We anticipate that the formation of the first CMS for DCM will greatly facilitate knowledge generation and knowledge translation in DCM by enabling clinicians and researchers to ‘speak a common language’ with regard to outcomes instruments. We hope that this set, which will focus on instruments in current use, will facilitate the standardised and comprehensive measurement of DCM and inspire a framework for the development and adoption of improved measures.
Ethics statements
Patient consent for publication
Acknowledgments
We thank the ongoing support of our wider stakeholders, including AO Spine RECODE DCM Community, and partners, including Myelopathy.org (DCM Charity; www.myelopathy.org). Further information about the initiative, and opportunities to get involved can be found at www.aospine.org/recode.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
BMD and AYT are joint first authors.
Twitter @AYanezTouzet, @angusgkmcnair
BMD and AYT contributed equally.
Collaborators The members of the AO Spine RECODE-DCM Steering Committee are: Evangeline Howard, Iwan Sadler, Ellen Sarewitz, Delphine Houlton, Julia Carter, Margot Miller, Theresa Brislin, Timothy F Boerger, Carla Salzman, Jillian Polasik, Shirley Widdop, Armin Curt, Sukhvinder Kalsi-Ryan, Anoushka Sing, Julio C Furlan, Chen Robert, Katherine Palmieri, Geno J Merli, James Milligan, Michelle Starkey, Michael G Fehlings, Brian K Kwon, Shekar Kurpad, Bizhan Aarabi, Vafa Rahimi Movaghar, James Harrop, James Guest, Mark R N Kotter, Benjamin M Davies, Jefferson R Wilson and Ricardo Rodrigues-Pinto.
Contributors BD was responsible for conceiving the article. AGKM contributed to the study design. BD and AYT wrote the protocol and manuscript and contributed equally to this paper. BK, MGF, MK and IS facilitated international collaboration. BD, AYT, ODM, KSL, DK, JCF, MGF, JSH, CMZ, RR-P, JM, ES, AC, VR-M, BA, TFB, LT, RC, JDG, SK-R, IS, SW, AGKM, MK provided critical appraisal of the manuscript. All authors critically revised and approved the manuscript.
Funding This work was supported by AO Spine through the AO Spine Knowledge Forum Spinal Cord Injury, a focused group of international Spinal Cord Injury experts. AO Spine is a clinical division of the AO Foundation, which is an independent, medically guided not-for-profit organisation. Study support was provided directly through the AO Spine Research Department. An award/grant number is not applicable.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.