Article Text
Abstract
Objectives Cure rate models accounting for cured and uncured patients, provide additional insights into long and short-term survival. We aim to evaluate the prognostic value of histological response and chemotherapy intensification on the cure fraction and progression-free survival (PFS) for the uncured patients.
Design Retrospective analysis of a randomised controlled trial, MRC BO06 (EORTC 80931).
Setting Population-based study but proposed methodology can be applied to other trial designs.
Participants A total of 497 patients with resectable highgrade osteosarcoma, of which 118 were excluded because chemotherapy was not started, histological response was not reported, abnormal dose was reported or had disease progression during treatment.
Intervention(s) Two regimens with the same anticipated cumulative dose (doxorubicin 6×75 mg/m2/week; cisplatin 6×100 mg/m2/week) over different time schedules: every 3 weeks in regimen-C and every 2 weeks in regimen-DI.
Primary and secondary outcome measures The primary outcome is PFS computed from end of treatment because cure, if it occurs, may happen at any time during treatment. A mixture cure model is used to study the effect of histological response and intensified chemotherapy on the cure status and PFS for the uncured patients.
Results Histological response is a strong prognostic factor for the cure status (OR 3.00, 95% CI 1.75 to 5.17), but it has no clear effect on PFS for the uncured patients (HR 0.78, –95% CI 0.53 to 1.16). The cure fractions are 55% (46%–63%) and 29% (22%–35%), respectively, among patients with good and poor histological response (GR, PR). The intensified regimen was associated with a higher cure fraction among PR (OR 1.90, 95% CI 0.93 to 3.89), with no evidence of effect for GR (OR 0.78, 95% CI 0.38 to 1.59).
Conclusions Accounting for cured patients is valuable in distinguishing the covariate effects on cure and PFS. Estimating cure chances based on these prognostic factors is relevant for counselling patients and can have an impact on treatment decisions.
Trial registration number ISRCTN86294690.
- sarcoma
- paediatric oncology
- statistics & research methods
- chemotherapy
- surgery
Data availability statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study are available from MRC BO06/EORTC 80931 collaborators and European Osteosarcoma Intergroup but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
By accounting for cured and uncured patients, this study provides additional insights into curing and life-prolonging effects of histological response and chemotherapy intensification.
In addition to survival probabilities for the entire cohort, as in the traditional framework, the method provides estimation of the cure fraction, that is, the chance that a given patient is cured, and of the progression-free survival for the uncured patients.
The method contributes to understanding the role of chemotherapy intensification, which is not clear when no distinction is made between curing and life-prolonging effects.
Since the actual received dose intensity differs considerably from the intended one as result of delays and reductions related to toxicity, the prognostic value of the treatment arm might not properly represent the prognostic value of the intensified chemotherapy.
The patients for whom histological response was not reported are excluded from the analysis.
Background
Osteosarcoma is a malignant bone tumour that occurs mostly in children, adolescents and young adults. Surgery alone is insufficient for curation of osteosarcoma patients and the introduction of adjuvant chemotherapy has led to significant improvements in survival.1 2 Current treatment for osteosarcoma includes neoadjuvant chemotherapy in combination with adequate surgery and different treatment schedules have been used in the past to establish an optimal chemotherapeutical design.3–5 Recently, in a randomised controlled international collaborative study, the European and American Osteosarcoma Study Group (EURAMOS) concluded that the three-drug regimen, consisting of methotrexate, adriamycin (doxorubicin) and cisplatin is the standard treatment. In the experimental arms no survival benefit of introducing Interferon-α−2b, an immune-modulator drug, for good responders (GR, <10% viable tumour cells after induction chemotherapy),6 or intensifying treatment by adding Ifosfamide and Etoposide for poor responders (PR, ≥10% viable tumour cells after induction chemotherapy)7 could be established. With respect to dose intensity, defined as given dose in mg/m2 per time unit,8 a phase-III study (BO06) in osteosarcoma that randomised patients between a conventional dose-intensity (CI) regimen or dose-intensified (DI) regimen with the same drug combination (doxorubicin plus cisplatin) in both arms, showed no difference in outcome.9 Despite the fact that the group of patients with the DI-regimen had a higher proportion of GR, no better event free or overall survival was seen in this group.9 It is noteworthy that histological response, as assessed by histological examination of the surgical specimen, is used as a key prognostic factor for survival of patients with osteosarcoma. Among PR, 5-year progression-free survival (PFS) is around 50% and among GR it is over 70%.7 10 However, histological response has been a debate as a surrogate marker for outcome in osteosarcoma.6 9–11 So, if even this actor is considered to be important, its interpretation is difficult, particularly in relationship to DI, and it is of interest to understand how this, and other factors contribute to better survival of PR.
In osteosarcoma, there is an ongoing debate about the effect of doses and dose intensities,4 9 12–14 which is an issue for other cancer types as well.15–22 All these studies have identified the presence of long-term disease-free survivors among osteosarcoma patients, who are practically considered as ‘cured’. Late relapse after 5 years follow-up occurs only in less than 5% of the patients.23 24 This suggests, at the moment that primary treatment is completed, a patient actually belongs to one of two categories: the cured ones and the uncured, who will experience disease progression within their lifetime. However, the identification of cured patients can only be done after a patient has been observed to remain disease-free for many years after treatment. In particular for patients alive and progression-free who have only been followed for a few years, the category to which they belong is not known. Hence, all the patients are usually studied together as one group. However, in the presence of a significant fraction of cured patients, the effect of a treatment or other prognostic factors may relate either to the probability of never experiencing progression or to the time free from progression for the uncured. This distinction is not captured by the traditional methods, that is, it is not possible to identify whether survival has improved since the treatment is curing more patients or because the uncured are living longer. As a result, certain effects might not be identified.
For this reason, cure rate models have started to be adopted in oncological studies as an alternative statistical modelling approach, which by accounting for cured and uncured patients, can provide additional insights into long and short term survival patterns.25–31 Through these models, it is possible to identify prognostic factors with a cure or a life-prolonging effect.25 32 The mixture cure model simultaneously studies these effects for the combined group of cured and uncured patients by assuming a regression model (typically logistic) for the effect of the prognostic factors on the cure probability and a regression model (eg, Cox) for the PFS of the uncured patients. Hence, compared with commonly used statistical techniques such as the Cox regression model that assume the same model for the survival of all patients, the cure model consists of two components (eg, logistic and Cox). This allows us to estimate not only survival probabilities as in the traditional framework, but also a cure fraction, the chance that a given patient is cured, and the PFS for the uncured patients. Such information may be used to select patients at high risk of progression for more aggressive chemotherapeutical strategies and protect those with high chances of being cured from the toxic side effects.
In this study, the BO06 clinical trial9 is revisited from a new point of view focusing on two different survival outcomes: the cure fraction and the PFS for the uncured patients. By accounting for cured patients, the prognostic value of histological response and intensification of chemotherapy is evaluated on each of these outcomes with the aim to reveal new insights into the complex nature of such effects.
Methods
Patients and chemotherapy information
Data from the MRC BO06/EORTC 80931 randomised controlled trial (RCT) for patients with localised resectable high-grade osteosarcoma, diagnosed between May 1993 and September 2002 were considered.9 Patients were randomly assigned to the conventional two-drug regimen (Reg-C), consisting of six 3-week cycles of doxorubicin (DOX, 75 mg/m2) and cisplatin (CDDP, 100 mg/m2) or to the DI regimen (Reg-DI), consisting of same doses administered 2-weekly and supported by G-CSF (5 µg/kg daily). The 2-week cycles of Reg-DI correspond to increasing the planned dose intensity of DOX and CDDP by a factor 1.5 compared with the conventional 3-week cycles. For both groups, surgery was scheduled at week 6 since the start of treatment, that is, after two cycles for Reg-C and after 3 cycles for Reg-DI, and postoperative chemotherapy was intended to resume 3 weeks after surgery. However, the received course of chemotherapy was often different from the intended one as a result of delays and reductions related to treatment-induced toxicity. The dataset consists of 497 patients (245 assigned to Reg-C and 252 to Reg-DI) aged 40 years or less. More details about the patients and chemotherapy can be found in the primary publication of the trial.9
Patient involvement
There was no patient or public involvement in the original RCT, which was run before this was recognised as good practice, nor in this study; patient and public involvement is not common in methodological studies.
Sample selection and follow-up
The outcome of interest is progression-free survival (PFS), that is, time free from any relapse (including distant metastasis) or death. PFS is computed from end of treatment because cure, if it occurs, may happen at any time during treatment and assuming that patients are either cured or not at early stages postdiagnosis it would not be appropriate. In addition, covariates such as histological response and received dose or DI are not known yet at time of randomisation. End of treatment is computed from the starting date of the last chemo cycle received, by adding the planned duration of the last cycle (14 or 21 days depending on the treatment arm). If the first follow-up visit after treatment occurs before this date, the first follow-up visit is considered as end of treatment (meaning that the treatment was interrupted or completed earlier than planned). For patients who do not receive any additional chemotherapy after surgery the date of surgery is considered as the end of treatment. Patients who did not receive chemotherapy, reported an abnormal dosage of one or both agents (more than 1.25×prescribed dose),13 did not have surgery or histological response was not reported, died or had disease progression during the treatment period, were excluded from the original dataset. The consort diagram is given in figure 1. A total of 379 patients were included in the analysis. Since the focus of the study is investigating cure or cancer progression after primary treatment, the overall survival is not further considered in this study because it incorporates also the effects of further treatments after cancer relapse.
CONSORT diagram. DI, dose-intensified.
Statistical analysis
A mixture cure model25 33–35 is used to assess the association of several variables of interest with cure and survival time for the uncured patients. This model assumes that the probability of survival without progression until time  given covariates x and
given covariates x and  is
is
 (1)
(1)
where 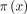 and
and  denote the probability of being cured given the covariate x and the probability of survival without progression until time t given the covariate
denote the probability of being cured given the covariate x and the probability of survival without progression until time t given the covariate  if the patient is not cured. The covariates of the two components in the mixture cure model can be the same or different, allowing for certain prognostic factors to have an affect only on one of these two outcomes. The probability of being cured
if the patient is not cured. The covariates of the two components in the mixture cure model can be the same or different, allowing for certain prognostic factors to have an affect only on one of these two outcomes. The probability of being cured  is modelled by logistic regression and the PFS for the uncured patients
is modelled by logistic regression and the PFS for the uncured patients  is modelled by a Cox proportional hazards model.36–38 The proportional hazards assumption for the uncured was assessed through a Kolmogorov-type supremum test based on martingale residuals,39 p values for the estimated univariate and multivariate models were 0.8, 0.85 and 0.71 respectively. A more detailed description about the model is provided in online supplemental material. We used the R package smcure40 (V.2.0) to compute the estimates of the parameters and their standard errors in the R statistical software environment.41
is modelled by a Cox proportional hazards model.36–38 The proportional hazards assumption for the uncured was assessed through a Kolmogorov-type supremum test based on martingale residuals,39 p values for the estimated univariate and multivariate models were 0.8, 0.85 and 0.71 respectively. A more detailed description about the model is provided in online supplemental material. We used the R package smcure40 (V.2.0) to compute the estimates of the parameters and their standard errors in the R statistical software environment.41
Supplemental material
The prognostic values of histological response, allocated treatment and received DI are evaluated through univariate and multivariate mixture cure models. The effects of the variables on the cure fraction and on the PFS for the uncured patients are summarised through ORs and HRs respectively. An OR larger than 1 means higher cure fraction with respect to the reference category, while a HR of less than 1 indicates a reduced risk for the uncured patients (longer PFS). In addition, the Wald-type CIs are computed at the 95% level. For visual inspection, Kaplan-Meier curves from end of treatment for the whole population and stratified according to histological response and treatment group are also reported.
Results
Based on the reverse Kaplan-Meier method,42 median follow-up time was 60 months (quartiles 52–67 months, range 0–116 months) from end of treatment. A total of 105 patients (57%) from Reg-C and 96 (49%) from Reg-DI experienced cancer relapse or death (all deaths were cancer related) during the follow-up period. Only three patients (1%) experienced disease progression after 5 years. The largest observed progression time was 5.3 and 7.7 years in Reg-C and Reg-DI, respectively. The estimated PFS for all patients (figure 2A) reaches a plateau around 7 years or earlier, indicating a consistent fraction of cured patients. There are 178 (47%) censored patients who were reported alive with no progression at the last contact. In total 159 patients (93%) from Reg-C and 172 (88%) from Reg-DI received the complete 6 chemotherapy cycles, while 19 patients (9 and 10 from Reg-C and Reg-DI respectively) did not receive any chemotherapy after surgery. GR was observed in 68 (37%) and in 97 (50%) Reg-C and Reg-DI patients, respectively. The Kaplan-Meier estimator from end of treatment, stratified by histological response, is shown in figure 2B.
(A) PFS for all patients. (B) PFS according to histological response. (C) PFS according to the allocated treatment. (D) PFS according to the allocated treatment and histological response. DI, dose-intensified; PFS, progression-free survival.
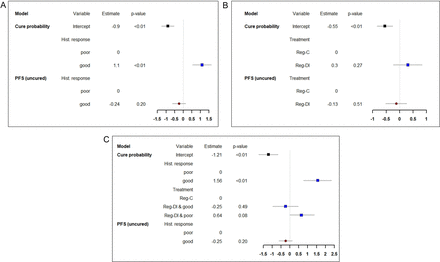
The estimated parameters together with 95% CIs and p values for a univariable logistic/Cox mixture cure model based on histological response are given in figure 3A. Histological response is found to be strongly prognostic for the cure fraction (OR 3.00, 95% CI 1.75 to 5.17) in favour of GR. For the uncured patients, there is no clear indication that GR is associated with longer PFS (HR 0.78, 95% CI 0.53 to 1.16). The estimated cure fractions and PFS at three or 5 years for the uncured patients with GR or PR are shown in table 1. Apart from separating the long-term from the short-term effect, the mixture cure model provides estimation of PFS for cured and uncured patients combined, as usually done in survival analysis. The estimated 3-year and 5-year combined PFS are given in the last two rows of table 1.
The estimated cure fractions, PFS at 3 and 5 years for the uncured patients, PFS at 3 and 5 years for cured and uncured patients combined according to histological response
Parameter estimates, 95% CIs and p values for the fitted univariate cure models based on histological response (A), allocated treatment (B) and the multivariate cure model (C). For each univariate or multivariate model, coefficients corresponding to the variables included in the cure probability model and in the PFS model for the uncured, are estimated jointly. Blue squares and red circles represent logORs and logHRs respectively. DI, dose-intensified; PFS, progression-free survival.
Parameter estimates together with 95% CIs and p values for a univariable logistic/Cox mixture cure model based on the allocated treatment are given in figure 3B. There is no evidence of a statistically significant effect of the allocated regimen on the cure fraction (OR 1.35, 95% CI 0.80 to 2.29, reference Reg-C). Among the uncured patients, the HR is 0.88 (95% CI 0.59 to 1.30) in favour of Reg-DI. These estimates suggest that the intensified treatment might be associated with better cure chances and longer PFS, but the CIs are too wide for clear conclusions in both components.
A multivariable cure model is also considered to assess the effect of the treatment group after conditioning on histological response. A logistic/Cox cure model was fitted with allocated treatment, histological response and an interaction term as covariates for the cure model. Only histological response was added to the survival model of the uncured patients to limit the number of parameters that need to be estimated. Parameter estimates together with 95% CIs and p values for the fitted model are provided in figure 3C. GR was found to be strongly associated with good chances of being cured as in the univariable model. Moreover, being allocated to the intensified treatment group (Reg-DI) seems to have a positive effect on the cure fraction among PR (OR 1.90, 95% CI 0.93 to 3.89) reference Reg-C). Among GR, there is no indication for such a positive effect (OR 0.78, 95% CI 0.38 to 1.59, reference Reg-C). The estimated cure fractions according to allocated treatment and histological response are given in figure 4. Slightly lower survival for the Reg-DI group versus the Reg-C group among patients with GR can also be observed in the stratified Kaplan-Meier estimator (figure 2D). For the uncured patients, GR is associated with better PFS (HR 0.78, 95% CI 0.54 to 1.13) compared with the reference group of PR, but not significantly. Estimated PFS curves for the uncured patients with GR and PR are given in figure 4. PFS probabilities at 3 and 5 years for the uncured patients are estimated from a univariable model and are as in table 1. Including also the allocated treatment group as a covariate for the survival model of the uncured patients did not show any evidence of an effect of treatment on PFS. A similar behaviour is observed if, instead of the allocated treatment, we use the received postoperative DI defined as in the primary analysis of the trial8 (details can be found in online supplemental material). This sensitivity analysis was performed since the effect of the preoperative treatment can already captured in the histological response. Prediction accuracy of cure probabilities from the multivariate model is assessed through a type of Brier score based on inverse probability of censoring weights43 (details can be found in online supplemental material).
Illustration of cure model interpretation. Cure chances are estimated on the bases of the allocated treatment and the observed histological response. In brackets the 95% CIs. PFS for the uncured patients is estimated based on histological response. DI, dose-intensified; PFS, progression-free survival.
Discussion
The aim of the MRC BO06/EORTC 80931 clinical trial was to investigate whether increasing the DI would improve the survival of patients with nonmetastatic limb osteosarcoma. The initial analysis of the trial9 showed that intensified chemotherapy results in higher chances of GR, mainly related to the increased number of cycles and amount of dose received before surgery. However, even though GR, as prognostic factor, is associated with better survival, no significant better survival was shown in the DI-regimen with a higher proportion of GRs, due to a more intensified treatment. This outcome makes the value of this marker debatable,11 44–46 or even better, makes the interpretation of this marker more complex. Here a new point of view is provided on the same data by using a cure model framework that distinguishes between short-term and long-term effects of covariates.
Previous studies had suggested that a fraction of osteosarcoma patients get cured by treatment, that is, do not experience cancer progression during their lifetime.8 10 However, as a result of censoring, it cannot be determined whether patients with relatively short follow-up and last observed alive without showing signs of the disease are cured or not. Compared with the traditional survival analysis methods, which estimate only the survival for the entire cohort, mixture cure models also allow us to estimate the chances of being cured and the PFS if a patient is not cured. From a clinical point of view, the cure fraction might be more informative than 5-year survival rates, in particular for young patients, being the most common patient group with osteosarcoma. Most importantly, enabling the investigation of separate covariate effects on cure and on PFS for the uncured patients, cure models provide a more detailed information of these effects.21 27 28
This study showed that the prognostic effect of histological response on survival is mainly a result of more patients being cured when they have GR. In other words, the chances of being cured after treatment for GR are considerably higher compared with those for patients with PR, while the effect of histological response on PFS for the uncured patients was not so clear. With a univariate analysis, there is no evidence of a significant effect of the allocated treatment on cure or on PFS for the uncured patients. However, in a multivariate model accounting for histological response and allowing for interaction between the treatment arm and histological response, Reg-DI seemed to have a positive effect on the cure fraction among PR, consistent with the findings of Bishop et al.10 The results suggest that, for GR the intensified treatment might have no effect on cure status, that is, GR in Reg-C do not have lower chances of being cured than GR in Reg-DI, which is also consistent with the findings of Bishop et al.10 One explanation might be the following. Since a GR in Reg-C is less common, given that it has been achieved suggests that these responding osteosarcoma cells do not reflect the self-renewing tumourigenic stem cell, at least less than in poor responsive osteosarcoma, hence have a different biological behaviour with higher chances of getting cured.45 Patients who respond poorly even despite high DI (Reg-DI) have less chemotherapy susceptible osteosarcoma cells with likely a different molecular profile, resulting in worse cure possibilities. In the initial analysis of the trial,9 a multivariate Cox regression model, accounting for histological response, did not find any significant effect for the treatment group or the interaction term. This might be due to a failure to separate short and long-term effect when cured and uncured patients are considered together as one group with the same survival pattern. Not accounting for the presence of cured patients hides some of the treatment effects. Here the highest cure fraction (59%) is observed among GR in Reg-C, while the lowest cure fraction (23%) corresponds to PR in Reg-C.
The focus of this study was on the prognostic value of histological response and allocated treatment to be in line with the focus of the initial analysis of the trial.9 Since the actual received DI differ considerably from the intended ones as result of delays and reductions related to toxicity,9 14 the prognostic value of the treatment arm might not properly represent the prognostic value of the intensified chemotherapy. Nevertheless, replacing the allocated treatment by the post-operative received DI gave similar results. It is however important to note that, in order to capture different effects on cure and short-term survival, mixture cure models require more parameters compared with the Cox regression model. As a result, they are less powerful in detecting a modest effect and CIs are wider. In addition, we emphasise that for an appropriate use of cure models, it is required to have a long follow-up for a considerable proportion of patients because otherwise it is not possible to correctly identify the cure fraction.33 38 In our study, this is confirmed by the plateau in the Kaplan-Meier survival estimator and the medical belief that patients surviving more than 5 years can be practically considered as cured. It would also be interesting to analyse other osteosarcoma datasets using the cure model in order to see whether the obtained results are consistent and yield a better understanding of the effect of different therapeutic options. We are working at the moment with the EURAMOS bone tumour clinical trial. The new insights provided by the mixture cure model are relevant for both patient and clinical perspective. They can help in identifying patients at a higher risk of progression, who might benefit from an intensified chemotherapy, and avoid that patients with good chances of being cured undergo a more toxic and aggressive treatment.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study are available from MRC BO06/EORTC 80931 collaborators and European Osteosarcoma Intergroup but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available.
Ethics statements
Patient consent for publication
Ethics approval
Permission to recruit patients to the MRC BO06/EORTC 80931 protocol was provided by the appropriate national and local regulatory and local committees. Link-anonymised data were used for the purposes of this study, and the use of the data was consistent with the consent taken. Because it involved retrospective review, the project was exempt from Human Subjects protection requirements by the Institutional Review Board of the European Organisation for the Research and Treatment of Cancer.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors EM and MF planned and designed the study. EM and MF conducted the statistical analysis and reported the results. EM, NvG, JA, HG and MF interpreted the results. EM drafted the first version of the manuscript. EM, NvG, JA, HG and MF: contributed to the manuscript drafting/revision and gave their final approval of the submitted version. MF acts as guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.