Article Text
Abstract
Objectives To explore intercountry and intracountry differences in physician opinions about continuous use of sedatives (CUS), and factors associated with their approval of CUS.
Settings Secondary analysis of a questionnaire study.
Participants Palliative care physicians in Germany (N=273), Italy (N=198), Japan (N=334) and the UK (N=111).
Primary and secondary outcome measures Physician approval for CUS in four situations, intention and treatment goal, how to use sedatives and beliefs about CUS.
Results There were no significant intercountry or intracountry differences in the degree of agreement with statements that (1) CUS is not necessary as suffering can always be relieved with other measures (mostly disagree); (2) intention of CUS is to alleviate suffering and (3) shortening the dying process is not intended. However, there were significant intercountry differences in agreement with statements that (1) CUS is acceptable for patients with longer survival or psychoexistential suffering; (2) decrease in consciousness is intended and (3) choice of neuroleptics or opioids. Acceptability of CUS for patients with longer survival or psychoexistential suffering and whether decrease in consciousness is intended also showed wide intracountry differences. Also, the proportion of physicians who agreed versus disagreed with the statement that CUS may not alleviate suffering adequately even in unresponsive patients, was approximately equal. Regression analyses revealed that both physician-related and country-related factors were independently associated with physicians’ approval of CUS.
Conclusion Variations in use of sedatives is due to both physician- and country-related factors, but palliative care physicians consistently agree on the value of sedatives to aid symptom control. Future research should focus on (1) whether sedatives should be used in patients with longer survival or with primarily psychoexistential suffering, (2) understanding physicians’ intentions and treatment goals, (3) efficacy of different drugs and (4) understanding the actual experiences of patients receiving CUS.
- palliative care
- adult palliative care
- medical ethics
Data availability statement
Data are available on reasonable request. The data are available from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
First international survey to compare the opinions of palliative care specialist physicians about continuous use of sedatives.
Relatively large number of participating physicians (near 1000), including different cultural backgrounds (Germany, Italy, Japan and UK).
All respondents are certified palliative care physicians with considerable clinical experience.
Use of a clear definition of continuous use of sedatives, not using ambiguous terms such as palliative sedation or continuous deep sedation.
Limitations include; not so high response rate (13%–70% in each country), measurement tools were not formally psychometrically validated.
Introduction
Terminally ill patients experience various distressing symptoms, which sometimes can be difficult to control without causing sedation.1 2 Sedation may be an unintended consequence of escalating doses of other symptom control medication, but sedative drugs can be used either intermittently to relieve transient distress or continuously for the relief of severe suffering refractory to standard palliative care measures.1–4 Because of the different ways in which sedatives are used, and the different ways in which sedation can be brought about, there is a lack of consistency in the terminology used to describe these different practices.5 6 Various terms related to continuous use of sedatives (CUS) have been employed, such as continuous deep sedation, rapid/sudden sedation and proportional/gradual sedation. However, regardless of how sedative use is described, it is apparent that sedative medications (ie, drugs given with the intention of promoting calm or inducing sleep) are frequently used to palliate severe symptoms in the last stages of far advanced illness.1–4
Empirical studies have reported both areas of agreement and divergent opinions about CUS across the world.5–9 Main controversies include: (1) appropriate indications for CUS (ie, whether deep sedation is appropriate for patients with longer survival or psychoexistential suffering), (2) physician intention and treatment goal (ie, whether it is legitimate for the physician to intend to produce a decrease in conscious level) and (3) choice of sedative and how it should be used. Our previous international survey of eight countries confirmed that CUS for physical and psychoexistential suffering in the last days of life is a generally accepted practice, while acceptability of CUS for psychoexistential suffering or for patients with a longer prognosis varied.7 However, statistical comparisons of physician opinions among and within countries as well as exploration of potential factors associated with physician approval were not pursued due to the sample heterogeneity and low numbers of responding physicians in some countries. In this secondary analysis, we focused on statistical comparisons and identifying determinants of physician approval of CUS using the opinions of palliative care physicians only in those countries with adequate number to be analysed.
The primary aim of this secondary analysis was to explore intercountry and intracountry variations in opinions of palliative care physicians about (1) approval of CUS, (2) intentions and treatment goals, (3) how to use sedatives and (4) beliefs about CUS. An additional aim was to explore the factors associated with their approval of CUS using both physician-level and country-level parameters. Our intention was to gain insights into the complexities surrounding CUS.
Methods
This was a secondary analysis of a questionnaire study in eight countries about medical practices and opinions of physicians regarding CUS.7 In the original survey, questionnaires were distributed to 8550 physicians in Belgium (n=555), Germany (n=1091), Italy (n=1083), Japan (n=734), the Netherlands (n=4000), Singapore (n=37), the UK (n=850) and the USA (n=200) between November 2018 and August 2019. For this study, we performed subgroup analysis on palliative care specialist physicians.
Subjects
Countries which had 100 or more responses from those who identified themselves as palliative care specialist physicians were included in this analysis. The numbers of responding palliative care specialist physicians in the original study were; 19 (Belgium), 273 (Germany), 198 (Italy), 334 (Japan), 0 (the Netherlands), 21 (Singapore), 111 (UK) and 22 (US); therefore, only data from Germany, Italy, Japan, and the UK were analysed for this report.
Procedure
Potential participants were identified by the respective national registries of certified palliative care physicians: the German Association for Palliative Medicine (Germany), the Italian Society of Palliative Care (Italy), the Japan Society of Palliative Medicine (Japan), and the Association for Palliative Medicine of Great Britain and Ireland (UK). Questionnaires were electronic in Germany, Italy and the UK; and were mailed in Japan. No financial incentive was used. Japanese physicians received two reminders.
Definition of sedation
We established the definition to be used in the questionnaire to avoid ambiguity in the interpretation and to describe practices used in all countries.7 In this study, CUS was defined as, ‘the CUS as a means to alleviate severe suffering in the last hours to days of life’. Continuous use was defined as, ‘either a continuous subcutaneous/intravenous infusion or a scheduled repeated injection’. No specific definition of sedatives was provided. It should be noted that the definition of CUS that was used related to the use of sedatives (rather than the achievement of sedation).
Development of the questionnaire
Due to the absence of validated questionnaires to quantify physician attitudes towards CUS, we developed our own questionnaire using expert opinion on the basis of literature review.7 Consensus on items for inclusion was reached following two face-to-face meetings and several subsequent rounds of email contacts among authors. The initial English version was translated into German, Italian, and Japanese. A pilot study with three physicians involved in the care of dying patients was conducted in all countries. Physicians in the pilots were asked to complete the questionnaire, and were interviewed afterwards to ascertain if the issues covered were applicable in their country, and to identify any missing domains. This resulted in minor adjustments to the English questionnaire. The final version was translated into German, Italian and Japanese.
The questionnaire contained 32 questions related to (1) physician approval for CUS in four situations, (2) intention and treatment goal, (3) how to use sedatives and (4) beliefs about CUS.
Physician approval for CUS
Physician approval of CUS was measured asking the degree of agreement with the statement ‘I consider the continuous use of sedatives as a means to alleviate severe physical/psychoexistential suffering in the last hours to days of life or for patients who are expected to live for at least several weeks an acceptable medical practice’, using a 5-point Likert type scale from 1 (strongly disagree) to 5 (strongly agree) for four situations: (1) physical suffering in the last hours to days, (2) psychoexistential suffering (in the absence of physical symptoms) in the last hours to days, (3) physical suffering for patients who are expected to live for at least several weeks and (4) psychoexistential suffering (in the absence of physical symptoms) for patients who are expected to live for at least several weeks.
Intention and treatment goal
Physician intention was measured by asking ‘what is your intention when you provide CUS in the last hours to days of life?’. Response options were (1) to relieve suffering, (2) to decrease the patient’s consciousness, (3) to induce unconsciousness and (4) to shorten the dying process, with a 5-point Likert type scale from 1 (never) to 5 (always) for each option. Moreover, physician-reported treatment goal was measured by asking two questions: ‘how often do you consider the goal of the continuous use of sedatives as a means to alleviate severe suffering in the last hours to days of life to have been achieved’, when (1) the patient is comfortable (not necessarily unconscious) and (2) the patient is unconscious, with each a 5-point Likert type scale from 1 (never) to 5 (always).
How to use sedatives
Physicians were asked, ‘How do you dose your medications when you provide the CUS as a means to alleviate severe suffering in the last hours to days of life?’. Response options were (1) ‘start low and gradually increase the dosage of the medications until the desired effect is reached’, and (2) ‘start sufficiently high in order to reach the desired effect rapidly’, with a 5-point Likert type scale from 1 (never) to 5 (always) for each option. Physicians were then asked about the medications they commonly used from a list of: midazolam, propofol, haloperidol, barbiturates, levomepromazine/chlorpromazine, opioids (with intent to provide sedation) and others. Answers were yes or no.
Beliefs about CUS
Physicians were asked about their degree of agreement with six statements about CUS, with response options being on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). Questions were; (1) ‘In my opinion, a competent patient with severe suffering has the right to demand the CUS in the last hours to days of life’, (2) ‘dying in a sleep through the CUS can be a good death’, (3) ‘the CUS in the last hours to days of life cannot sufficiently alleviate suffering in all patients, even when patients become unresponsive’, (4) ‘the CUS in the last hours to days of life shortens the duration of the dying process’, (5) ‘I feel that in clinical practice the CUS in the last hours to days of life can be difficult to distinguish from euthanasia’ and (6) ‘the CUS as a means to alleviate severe suffering in the last hours to days of life is not necessary, as suffering can always be relieved with other measures’.
Physician backgrounds
Physicians were asked to report their age, religion, self-identified specialty, work place, work experience, involvement in care of dying patients in last 12 months and experience with CUS.
Statistical analyses
Data were collected between March and December 2019. Data were imported into an SPSS template in each country and merged into a final dataset. To examine intercountry differences, we compared the variables using analysis of variance (ANOVA) with post hoc pairwise t-test, χ2 test or Cochran-Mantel-Haenszel test, where appropriate. A category of ‘others’ in the choice of sedatives was excluded from the analyses due to its lack of the clarity and the small number of the responses concerned (<12%). To examine intracountry difference, we visualised the distribution of the responses, and calculated kurtosis values (ie, excess kurtosis) and SD for each item. Excess kurtosis quantifies the shape of a distribution compared with the normal distribution. For a mesokurtic distribution, excess kurtosis is zero. Negative values indicate a platykurtic distribution (ie, wide variation in the responses). Due to lack of established cut-off points, we assumed that if kurtosis values were −0.4 or less, then responses showed a ‘wide’ intracountry difference in this analysis, with reference to interpretations of visual presentations (online supplemental figure 1). We decided not to use IQR for this aim, because the range of the responses were narrow. Finally, we performed logistic regression analyses using physician approval of CUS for two situations in which patients had prognoses of weeks or more; and using physicians’ background, country and six belief items as independent variables. The dependent variables were collapsed into two categories: agree and strongly agree versus others. As this report only concerns large differences, we determined statistically significant results as p values<0.001 (0.05/50) and effect sizes (ES)>0.60, with reference to the Bonferroni corrections (46 overall comparisons in this study). Statistical analyses were performed using IBM SPSS Statistics V.25.0.
Supplemental material
Supplemental material
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
A total of 3630, 1083, 734 and 850 questionnaires were distributed in Germany, Italy, Japan and the UK, respectively; and 546 (15%), 214 (20%), 513 (70%) and 114 (13%) participants returned effective responses (figure 1). Of these, the answers of 273, 198, 334 and 111 from palliative care specialist physicians were analysed for this report. Table 1 summarises respondents’ backgrounds. Across countries, the median age varied from 44 to 52 years, and median clinical experience from 20 to 27 years. Each physician cared for a median of 100–150 terminally ill patients per year. Less than 10% worked at hospitals in Italy and community palliative care services in Japan.
Backgrounds of the physicians
Participant flow. PC, palliative care.
Physician approval of CUS
The majority of respondents reported that they regarded CUS as acceptable for patients with refractory physical suffering in the last hours to days, and a substantial proportion also agreed that CUS is acceptable for refractory psychoexistential suffering in this setting, without intracountry differences (figure 2A). Italian specialists were significantly more likely to affirm that CUS was acceptable for psychoexistential suffering than Japanese and UK physicians (ES, 0.61–1.11; p<0.001; table 2). On the other hand, there were significant inter-country differences in physician approval for CUS in patients with predicted survival of weeks or more, and all four countries showed wide intracountry differences (figure 2B). Japanese and UK physicians were less likely to regard CUS as acceptable than German and Italian physicians (ES, 0.71–1.14; p<0.001; table 2).
Comparisons of physician opinions about continuous use of sedatives among UK, Japan, Ital and Germany
Intercountry and intracountry difference in physician approval as a medical practice of continuous use of sedatives. (A) Intercountry difference. (B) Intracountry difference. Mean scores were plotted (1: strongly disagree to 5: strongly agree), with bars indicating 95% CIs. Square dots mean items with ‘wide’ intracountry differences, defined as kurtosis values of −0.4 or less (ie, wide intracountry variations of the responses).
Intention and treatment goal of CUS
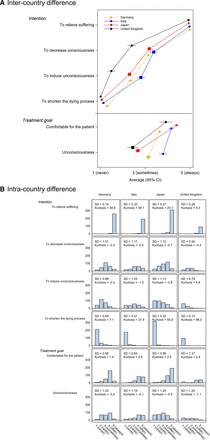
Almost all palliative care physicians from four countries reported that they performed CUS with the intention of alleviating suffering, without intending to shorten the dying process. There were neither intercountry nor intracountry differences (figure 3A,B). UK specialists were significantly less likely to report that their intention was to decrease level of consciousness or to bring about unconsciousness than physicians from Germany, Italy and Japan (ES, 0.74–1.18; p<0.001; table 2). Italian physicians were significantly more likely to report that the treatment goal was to achieve unconsciousness than the other three countries (ES, 0.60–1.14; p<0.01; table 2). There were, however, wide intracountry differences in one or more physicians’ statements about physician intention to decrease consciousness or to induce unconsciousness, and treatment goals in all four countries (figure 3B).
Intention and treatment goal of continuous use of sedatives. (A) Intercountry difference. (B) Intracountry difference. Mean scores were plotted (1: never to 5: always), with bars indicating 95% CIs. Square dots mean items with ‘wide’ intracountry differences, defined as kurtosis values of −0.4 or less (ie, wide intracountry variations of the responses).
How to use sedatives
In all countries, most respondents indicated that, as a general principle, they usually started low and gradually increased the dosage of sedatives (often or always, 81%–92%) and that midazolam was the most frequently used medication (95%–98%), without significant intercountry difference. On the other hand, the number of physicians who reported that they usually started with high doses was significantly greater in Germany and Italy compared with Japan and the UK (table 3). Moreover, levomepromazine/chlorpromazine were significantly more frequently reported to be used by UK physicians, while opioids were significantly more frequently reported German and Italian physicians.
How to use sedatives
Beliefs about CUS
Almost all physicians disagreed with the statement that CUS is not necessary, as suffering can always be relieved with other measures, without intercountry or intracountry differences (figure 4A,B). For the statement that CUS may not alleviate suffering adequately even in unresponsive patients, there were wide intracountry differences in all countries, and the proportion of physicians who agreed vs disagreed was comparable (figure 4B).
Opinions about continuous use of sedatives. (A) Intercountry difference. (B) Intracountry difference. Mean scores were plotted (1: strongly disagree to 5: strongly agree), with bars indicating 95% CIs. Square dots mean items with ‘wide’ intracountry differences, defined as kurtosis values of −0.4 or less (ie, wide intracountry variations of the responses). CUS, continuous use of sedatives.
In the other items, there were some intercountry or intracountry differences observed: UK physicians were significantly less likely to state that a patient has the right to demand CUS, with wide intracountry difference (ES, 1.11–1.91; p<0.001; table 2); Japanese physicians were significantly less likely to regard dying in sleep as a result of sedation as a good death (ES, 0.83–1.51; p<0.001); Italian physicians were less likely and German physicians were more likely to believe that CUS shortens the dying process than physicians in the other three countries (ES, 0.68 and 0.65–0.66; p<0.001); and Italian physicians were significantly less likely to believe that CUS is a kind of euthanasia (ES, 0.63–0.89; p<0.001).
Factors associated with physician approval of CUS
Both physician and country factors were significantly associated with physician approval of CUS (table 4). Adding the country of the respondents as a variable into models incorporating physician factors improved R2. Physician beliefs such as ‘a competent patient has the right to demand CUS’ and ‘dying in a sleep induced by CUS can be a good death’ were associated with physician approval of CUS. After adjustment for physician backgrounds, compared with the UK, Japanese respondents showed significantly less approval (OR, 0.39–0.53, p<0.01) and German and Italian respondents showed significantly more approval (1.93–2.08 and 1.87–2.74, respectively, p<0.001) of CUS for patients with a prognosis of weeks or more.
Factors associated with physician approval of continuous use of sedatives in three scenarios
Discussion
This study revealed intercountry and intracountry differences about opinions about CUS among palliative care physicians in four countries, and found that the difference in physician approval of CUS was due to both physician differences and country differences. We found one area with which almost all palliative care physicians agreed (ie, where neither intercountry nor intracountry differences were observed), and five areas where there were large intercountry and/or intracountry differences.
One consistent finding across the countries was that almost all palliative care physicians agreed with the importance of CUS as a means to relieve severe suffering in the last hours to days of life. There were no intercountry and intracountry differences in disagreement with the statement that CUS is not necessary because suffering can always be relieved with other measures, or with agreement with the statement that the intention of sedative use is to relieve suffering and not to shorten the dying process. Midazolam was the most frequently reported sedative medication. These results are consistent with multiple national surveys with similar results,10–14 and many empirical studies have reported that a considerable number of patients require subcutaneous infusions of midazolam to obtain adequate symptom relief in the last days.3 7 It can be safely concluded that palliative care specialist physicians considered that CUS is a necessary and acceptable medical treatment for the relief of severe suffering in the last days of life.
On the other hand, there were at least five areas with wide intracountry and/or intercountry variations. One was in relation to whether palliative care physicians considered CUS to be acceptable for patients with longer survival (eg, at least several weeks). Physicians from Germany and Italy were more likely to regard CUS in this situation as acceptable than physicians in Japan and UK, despite wide intracountry variations observed in all four countries. Multivariate analyses identified that country and two physician beliefs related to patient autonomy (a competent patient has the right to demand CUS) and good death (dying in a sleep through CUS can be a good death) were major determinants of physician approval for CUS for such patients. Although CUS is usually reserved for patients close to death, variations in international responses in this study suggest a more heterogeneous attitude of respondents to this type of CUS.1–4 More in-depth research and discussion is needed to reach an intercountry and intracountry consensus if CUS is appropriate for patients with longer survival from cultural, philosophical, ethical and legal points of view.
Another divergence of opinion was about whether respondents considered psychoexistential suffering as an acceptable indication for CUS. Physicians from Germany and Italy were more likely to regard CUS for psychoexistential suffering as appropriate than Japanese and UK physicians, although absolute ratings were neutral. The findings are consistent with German studies that demonstrated increase in use of palliative sedation for psychoexistential suffering,14–16 and Italian statements that psychoexistential suffering can be an indication for palliative sedation as psychoexistential suffering cannot be clearly differentiated from physical suffering in patients close to death.17 18 On the other hand, Japanese guidelines regard palliative sedation for psychoexistential suffering as exceptional even when death is imminent, on the legal assumption that only physical suffering can be accepted if the use of sedatives has life-threatening potential.19 20 One explanation for this discrepancy may be differing interpretations of what is meant by the term, ‘psychoexistential suffering’. Recently some authors have proposed classifications of psychoexistential suffering, such as: agent-narrative suffering versus neurocognitive suffering21; or psychoexistential suffering developed during the disease trajectory as a reaction to the approaching of death vs a direct result of neurological symptoms versus pre-existing psychological problems22; or existential suffering (acute, subacute and chronic) versus psychiatric symptoms.23 Also, empirical studies have revealed that terminally ill patients experience a combination of physical and psychoexistential suffering; and CUS after failure of repeated intermittent sedation can be different from CUS as first-line treatment.24–27 Future research focus should try to better classify ‘psychoexistential suffering’ and understand the rationale for using CUS in patients with psychoexistential suffering.
The third area of disagreement was whether intentional reduction in consciousness, especially unconsciousness, was the intended goal of CUS. UK physicians were significantly less likely to report that this was their intention than German, Italian and Japanese respondents. Moreover, German and Italian physicians were significantly more likely to report that they often started with high dose of sedatives than Japanese and UK physicians. This is in line with empirical studies and expert perspectives that UK and US palliative care physicians stress the proportional use of sedatives as a measure of symptom control; and disagree that the primary purpose of sedative use at the end of life is to decrease consciousness.28–33 The ethical principle of double effect has a strong ethical and legal foundation in UK and USA.32–35 As applied to the practice of CUS, the relief of suffering is the primary purpose of using drugs with sedative effects and sedation is a foreseeable but unintended consequence thereof. If it was possible to achieve the same effect without causing sedation then this would be preferable. In Germany and Japan (whose criminal laws are based on the same foundation), the principle of double effect is not a fundamental principle and intention to decrease consciousness can be allowed if it is appropriate as a medical act. In Italy, the national ethics committee approves a fundamental right for patients to be relieved from suffering close to death by inducing sleep, followed by the legalisation.17 18 Whether decrease in consciousness should be an appropriate primary intention or treatment goal should be further discussed.
A fourth area with large intercountry difference was the selection of sedatives. This study found that levomepromazine/chlorpromazine was often reported as being used as sedatives, especially in the UK (87%) compared with other countries (10%–29%). Levomepromazine is used for control of nausea and in higher doses for agitation, although empirical evidence is limited.32 33 36 The role of levomepromazine in alleviating refractory symptoms, especially agitated delirium, should be explored in clinical trials or well-designed observational studies. In Germany and Italy, more palliative care physicians listed opioids as a medication used for their sedative effect, while clinical guidelines do not generally recommend the use of opioids in CUS because of increased opioid toxicity, but rather describe their adjuvant use for control of pain and dyspnoea.1 2 The reason for the high reported use of opioids for sedation among German and Italian respondents is unclear, and this study did not specifically aim to investigate the use of opioids in CUS. One study indicated that midazolam may be unavailable in some home care settings in Italy.37 Relative benefits and burdens of these medications need to be studied.
The last area of divergent opinion is whether one can be sure that CUS can sufficiently alleviate suffering even when patients become unresponsive. In all countries surveyed, there were wide intracountry variations in agreement with the statement about the degree to which patients receiving sedatives actually achieve symptom relief. This finding is consistent with the opinions of some experts that there is a need for accurate measures of symptom relief for sedated patients.38–41 Approaches such as Bispectral Index monitoring are in the early stages of evaluation as measures to monitor sedative effects in palliative care patients.42 43 Other research approaches may include the use of functional MRI, which has also been used to assess awareness in patients in vegetative or minimally conscious states.44–46 More research is needed to understand real patient experience, including the degree of distress in patients with reduced consciousness.
One of the major strengths of this study was the relatively large number of participating physicians (near 1000), and all were palliative care physicians. Our questionnaire used a clear definition of CUS and underwent pilot testing and modification before being used. However, there were several important limitations. First, the questionnaire used was not formally psychometrically validated; especially, the concept of intention and treatment goal may be more complex, and no specific definition of sedatives was provided, although we made considerable efforts to maximise the face validity of the questionnaire. Second, the response rates were <50% in three countries while being exceptionally high in Japan, and we could not compare responding and non-responding physicians; therefore, we cannot know whether the respondents’ views were representative, and the differences in response rates might have had some influence. Palliative care specialist physicians were self-identified, and difficulties in obtaining (sub)specialty certification varies among the countries. Third, the analyses reported in this paper were exploratory and post hoc rather than related to the testing of preplanned hypotheses and so should be interpreted with caution. Fourth, R2 values in the regression models were not so high, indicating important unaccounted and unmeasured factors. Fifthly, we investigated physicians’ attitudes in hypothetical situations and direct application of the findings to clinical situations is limited; for example, prognostication is always to some degree uncertain in real clinical settings. Finally, the results relate to the four countries descried and are not generalisable to other countries, as not all countries have a certification system for palliative care physicians.
Conclusions and implications
This study identified similarities and differences in opinions of palliative care physicians in four countries about CUS. Palliative care physicians agreed that; CUS for symptom relief is a necessary and acceptable medical treatment for the relief of suffering in the last days of life. On the other hand, there were wide variations of opinions about (1) whether CUS is acceptable for patients with longer survival or psychoexistential suffering, (2) whether physicians prescribing CUS intend to decrease consciousness, (3) use of levomepromazine as sedatives and (4) whether CUS sufficiently alleviates suffering when patients become unconscious. These results suggest further lines of clinical research for the future. In clinical practice, an individualised, case-by-case approach should be adopted in any situation where CUS is considered, as initiation of CUS is a complex process.
Data availability statement
Data are available on reasonable request. The data are available from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
The study protocol was approved by ethics committees in Germany (Medizinischen Fakultät, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU),123_18 B); Japan (Seirei Mikatahara General Hospital, 18-58); and the UK (University College London, NA). Ethics committee approval was not required according to national policies in Italy.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors TM and JR developed the concept of this study; TK, DH and MTH performed or supervised statistical analysis and data synthesis; and PS, NS, GM, CK, SS, LD, MM, MH, LR, and LK contributed to interpretation of data. TM, PS and NS drafted the manuscript, and TK, GM, CK, SS, DH, LD, MTH, MM, MH, LR, LK and JR revised it critically for important intellectual content. All authors approved the final version of the manuscript. TM has full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding This study was funded by the Japan Society for the Promotion of Science (JSPS), KAKENHI, 19H03869 and 19K10575.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.