Article Text
Abstract
Objective Anorectal melanoma (AM) is a rare but aggressive tumour with limited information in the existing literature. This study aimed to assess the effect of surgical treatment for AM and predict the prognosis of affected patients.
Design A retrospective cohort study.
Setting Data of patients diagnosed with AM between 1975 and 2016 in the USA were collected from the Surveillance, Epidemiology, and End Results (SEER) database.
Participants This study enrolled a total of 795 patients with AM from the SEER database and the validation cohort comprised 40 patients with AM enrolled from Chinese institutes.
Primary and secondary outcome measures Overall survival (OS) and AM-specific survival (AM-SS).
Results A total of 795 patients with AM diagnosed between 1975 and 2016 were enrolled in this study. Data over the past four decades showed a trend of increase in incidence rate. A nomogram based on a multivariate Cox regression model was generated to predict AM-SS. The C-index of the nomogram was 0.74 (95% CI 0.71 to 0.77) on internal verification. In the validation cohort, the C-index of the nomogram was 0.72 (95% CI 0.68 to 0.76). The results of propensity score matching (PSM) analysis showed that patients who underwent surgical treatment achieved significant survival (OS: log-rank=17.41, p<0.001; AM-SS: log-rank=14.55, p<0.001). Patients who underwent surgery were stratified into local and extended surgery subgroups. AM-SS and OS were also compared after PSM, but the results were not significantly different between the two surgery subgroups (all p>0.05).
Conclusions The nomogram based on the analysis of SEER data showed good performance in predicting OS and AM-SS. Patients with AM can benefit from surgery; however, extensive surgery and appendectomy may not improve AM-SS or OS.
- anorectal melanoma
- nomogram
- overall survival
- surveillance, epidemiology, and end results
Data availability statement
Public data of this study are available from the SEER database (https://seer.cancer.gov). The validation data are from our hospital and available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The study explored the effect of prognostic factors on overall survival and developed a nomogram to predict the 5-year dynamic death rate of patients with anorectal melanoma.
The study compared the overall survival between patients who did and did not undergo surgery and used propensity score matching analysis to eliminate bias, and confirmed that extensive surgery and lymphadenectomy did not improve survival in patients with primary anorectal melanoma.
Study limitations included the rarity of the disease which resulted in enrolment of an insufficient number of patients and potential misclassification of histological data from the Surveillance, Epidemiology, and End Results (SEER) database.
The change in the incidence of anorectal melanoma may be an artefact caused by recording or coverage of the SEER database, which may make the conclusion less credible.
Background
Anorectal melanoma (AM) is a subtype of mucosal melanoma that originates from the sinonasal, anorectal and genitourinary mucosa and has a dismal prognosis.1–3 It accounts for about 1.5% of all melanoma cases and has an incidence of about 2.7 patients per 10 million population per year in the USA.4 5 However, due to its low incidence and the lack of clinical information, a standardised treatment for AM is lacking.6 AM is likely to remain unnoticed and diagnosed at an advanced stage due to its non-specific symptoms. Therefore, AM has become an aggressive subtype of melanoma, with a 5-year overall survival (OS) rate of 14%–20%.7
The survival rate of some patients with AM has recently increased due to the development of targeted therapies and immunotherapy.2 8 9 Nevertheless, surgical resection remains the most effective therapy for patients with AM. However, patients with AM with distant metastases may not gain significant survival benefits from surgery, and the standard operative area for resection and lymph node (LN) dissection is controversial.10 11 Surgical treatments generally include limited resection (LR) and extended resection (ER). ER refers to tumour resection and LN removal, while LR refers to tumour resection without LN dissection. Compared with LR, ER may control the lymphatic spread of melanoma and result in lower local relapse rates, but can also result in prolonged hospital stays, injury and a low quality of life.
The search for more effective prognostic models for AM is limited due to the rarity of the disease.12 Most recent evidence of AM relies only on small case series from single institutions.13–15 Therefore, we investigated the effect of surgery on patients with AM from the Surveillance, Epidemiology, and End Results (SEER) database, which includes information from a large population.
Materials and methods
Data source
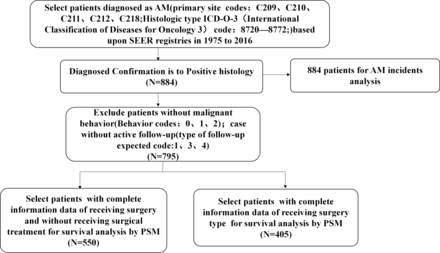
This retrospective review analysed all patients with AM enrolled in the SEER database who were diagnosed between 1975 and 2016. The SEER programme includes a large public cancer database of patients from the USA and is updated annually.16 A total of 795 patients diagnosed with AM were selected for OS and AM-specific survival (AM-SS) analyses based on the following criteria: cases with primary site codes (rectum or anus) of C209, C210, C211, C212 and C218; and ICD-O-3 (International Classification of Diseases for Oncology 3) histological type code for melanoma of 8720–8772. Patients without a positive histological confirmation of AM were excluded. Patients without malignant tumour behaviour (behaviour codes: 0, 1 and 2) or without active follow-up (type of follow-up expected codes: 1, 3 and 4) were also excluded. The results of the selection process are shown in figure 1.
Flow chart of inclusion and exclusion criteria of patients from the SEER database. AM, anorectal melanoma; PSM, propensity score matching; SEER, Surveillance, Epidemiology, and End Results.
Patients were stratified by surgery type into LR and ER group. Due to the longitudinal study duration, different encoding methods found in the SEER database were followed. The codes for the two groups are presented in online supplemental table 1. In addition, the SEER historical classification of stage of disease was used because it did not change over time and allowed a much greater number of patients to be enlisted. The stage of AM included localised, regional and distant. A tumour limited to the mucosa or submucosa (superficial invasion) was classified as localised. The spread of AM was classified as regional if the tumour spread to the ischiorectal fat or tissue, perianal skin, perineum, rectal mucosa or submucosa, skeletal muscles (external anal sphincter, levator ani), subcutaneous perianal tissue, and vulva. A distant disease is considered when AM spread beyond the abovementioned limits.
Supplemental material
Validation cohort
Due to the rarity of this malignant disease, the validation cohort was selected from two Chinese institutes—Xiangya Hospital of Central South University and Hunan Provincial People’s Hospital. A total of 40 patients diagnosed between 2014 and 2020 who met the abovementioned criteria and the standards of the hospital’s ethics committee were approved for enrolment in this retrospective study.
Nomogram and validation
The nomogram includes all significant prognostic factors in the Cox regression model based on the SEER database by using the rms package in R V.2.1.1. According to the different classifications of prognostic factors. According to the different classification of each feature, project up to the small scale (points) to get the score of each item. The higher the score, the worse the survival prognosis; the total score is obtained by adding the scores. The total points can be projected downwards to obtain the patient’s survival rate. The nomogram was internally validated in the SEER cohort and externally validated in the validation cohort. The C-index was used to evaluate the discriminative ability of the nomogram, which showed a relatively good discriminative ability between 0.71 and 0.90. The calibration plot was also used to evaluate the performance of the nomogram. In a perfectly calibrated model, the predictions should fall at a diagonal 45° line in the calibration plot.
Propensity score matching
Propensity score matching (PSM) is an accurate way to avoid bias when comparing the outcomes of two groups. This study aimed to provide evidence that can assist with clinical decision-making. Herein, we analysed patients’ prognosis after different surgical treatments using PSM.
Statistical analysis
Patients’ clinical characteristics were summarised with descriptive statistics using SPSS V.24.0. The incidence of AM was adjusted to the 2000 US standard population (19 age groups: Census P25-1130). Univariate and multivariate models were generated to identify the factors that correlated with AM-SS. AM-SS was defined as patients’ survival time between initial diagnosis and AM-specific death. The survival curves for OS and AM-SS were plotted using Kaplan-Meier analysis by log-rank test.17
A nomogram was established based on the results of the univariate Cox proportional hazards model from the SEER cohort, combining all independent prognostic factors to predict 1-year, 3-year and 5-year AM-SS using the rms package in R V.2.1.1 software (http://www.r-project.org/). PSM was used to match patients with similar baseline variables.18 The propensity score was based on the logistic regression model.19 Matching was performed using a 1:1 matching protocol without replacement, and standardised differences were controlled to less than 10%. The OS and AM-SS were analysed after PSM.
Patient and public involvement
Neither patients nor the public were involved in the design, conduct, reporting or dissemination plans of this research.
Results
Incidence
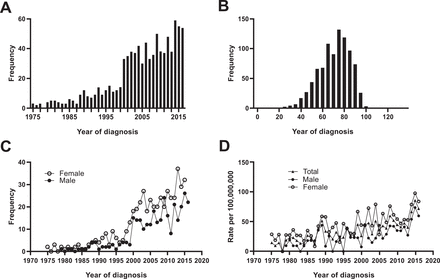
A total of 795 patients diagnosed between 1975 and 2016 were selected from the SEER incidence database. The number of patients diagnosed with AM increased annually in the SEER database (figure 2A). Age at diagnosis ranged from 59 to 80 years and the median age at diagnosis was 71 years (figure 2B). Within this trend, the number of new female patients diagnosed with AM increased compared with male patients, according to the SEER database (figure 2C). The age-adjusted incidence of this disease significantly increased over time, and the incidence of AM has exceeded 0.5 per million in recent decades (figure 2D). This may indicate a trend of increasing incidence over the past few decades.
Incidence of anorectal melanoma (AM): (A) number of new patients diagnosed with AM between 1975 and 2016; (B) distribution of age at AM diagnosis; (C) growth trend of AM between male and female patients; (D) age-adjusted incidence rate increasing over time.
Clinicopathological characteristics and survival
A total of 795 patients with AM were selected from the SEER cohort according to our exclusion criteria. The median age of this cohort was 71 years, with 221 patients (28%) older than 78 years. This cut-off age was obtained using the X-tile software as patients over 78 years of age had poor OS and AM-SS (online supplemental figure 1A,B). Among the SEER cohort, the majority (83%) were white patients, and almost 44.2% (n=630) of the patients died and about 41.8% (n=426) of cases died of this malignant tumour (table 1). Similarly, 41 patients who underwent surgery at our institutes were enrolled in the validation cohort.
Supplemental material
Clinicopathological characteristics of the SEER and validation cohorts
Associations between clinicopathological parameters and disease-specific survival
We considered the factors associated with OS and AM-SS for analysis. A log-rank test analysis of the SEER cohort showed that patients with LN positivity (online supplemental figure 1C,D) and distant SEER stage at presentation (online supplemental 1E,F) had poor OS and AM-SS. We also performed univariate and multivariate analyses to identify the clinical prognostic factors for AM. The univariate Cox regression analysis among the SEER cohort indicated that age at diagnosis, location, stage, LN positivity and chemotherapy were significantly associated with AM-SS (p<0.05; table 2).
Univariate and multivariable Cox proportional hazards models of anorectal melanoma-specific survival
In the multivariable Cox regression model, patients over 78 years of age had a 1.41-fold increase in the odds of AM-specific mortality (HR, 1.41; 95% CI 1.12 to 1.78; p=0.004). Compared with patients with AM whose primary tumour site was rectal, those with anal melanoma had better prognosis (HR, 0.81; 95% CI 0.65 to 1.01; p<0.05). In addition, patients in the SEER cohort with distant stage (HR, 3.37; 95% CI 2.56 to 3.42; p=0.000) and one or more positive LN (HR, 1.71; 95% CI 1.14 to 2.57; p=0.017) may be associated with increased AM-specific mortality (table 2).
Nomogram for AM-SS of the SEER and validation cohorts
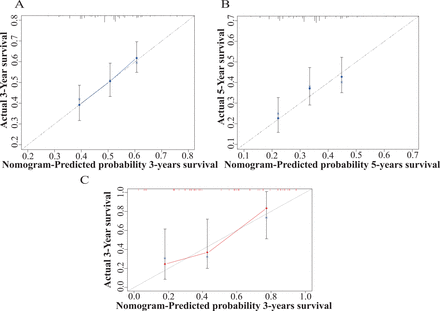
We built a nomogram based on the multivariable analysis to identify potential predictors of AM-SS using R Bioconductor (figure 3). Age at diagnosis, location, stage and LN positivity were included in the nomogram. The nomogram includes the risk factors for predicting the 1-year, 3-year and 5-year AM-SS of patients with AM. We also conducted a validation study using the SEER cohort for internal verification. The C-index of the nomogram on internal verification was 0.74 (95% CI 0.71 to 0.77) (figure 4A,B). The C-index was 0.72 (95% CI 0.68 to 0.76) when we applied the nomogram to predict AM-SS in the validation cohort. It is well known that a C-index that exceeds 0.7 means that the established nomogram is reliable20 (figure 4C).
Nomogram for predicting anorectal melanoma-specific survival among patients from the SEER cohort. LNs, lymph nodes; SEER, Surveillance, Epidemiology, and End Results.
Calibration curve of the nomogram for patients in the SEER cohort and validation cohort. (A and B) Bootstrap validation of the prognostic nomogram at 3-year and 5-year survival in the SEER cohort. (C) Bootstrap validation of the prognostic nomogram at 3-year survival in the validation cohort using 40 patients. The predicted probability of the nomogram for overall survival is on the x-axis, while the actual overall survival is on the y-axis. SEER, Surveillance, Epidemiology, and End Results.
Surgical treatment and type of AM after PSM
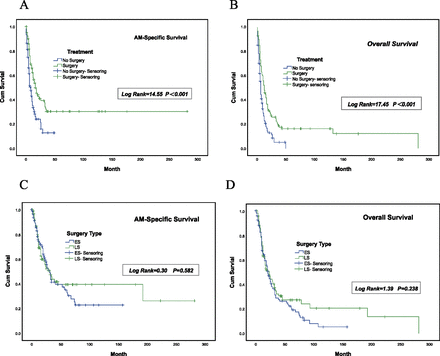
To evaluate the prognostic value of surgery in patients with AM, we identified 550 patients for whom complete surgery code data were available. Before PSM, we found differences between the surgery and non-surgery groups. PSM was used to eliminate intergroup bias. We set the calliper width to 0.02 after 1:1 matching. A total of 204 patients were registered in this study (online supplemental table 2). We compared the AM-SS and OS using Kaplan-Meier analysis and found that patients who underwent surgical treatment achieved significantly better survival than those who did not undergo surgery (OS: log-rank=17.41, p<0.001; AM-SS: log-rank=14.55, p<0.001; figure 5A,B).
Supplemental material
Overall survival and AM-specific survival stratified by Kaplan-Meier analysis and log-rank test according to (A and B) patients with or without undergoing surgery and (C and D) surgery type. AM, anorectal melanoma; ES,extended surgery; LS,limited surgery.
In addition, because surgical type also affects prognosis, we divided the cohort of patients who underwent surgery into LR and ER subgroups according to surgery type. We set the calliper width to 0.005 after 1:1 matching. Only small intergroup differences were observed (online supplemental table 3). We also compared AM-SS and OS using Kaplan-Meier analysis and found no significant intergroup differences, indicating that extensive surgery and lymphadenectomy did not improve survival in patients with AM (p>0.05; figure 5C,D).
Supplemental material
Discussion
Primary AM is the third most common site for primary mucosal melanoma after the head and neck and vulvovaginal regions.21 22 Distant metastasis in the early stage makes the treatment and diagnosis of primary AM ineffective.23 Radical surgery seems to be the best treatment for patients with AM, while optimal surgical strategies are also vital to improving the OS of patients with AM.24 25 However, there is a long-standing debate regarding the scope of surgery in patients with AM with distant metastasis.26 27 A few retrospective studies recently reported that patients with AM failed to achieve survival benefits from extensive surgery.28–30 Moreover, due to the rarity of AM, its prognostic classification has remained challenging for many years.31 This study aimed to investigate the prognostic trends of AM and build more accurate prognosis prediction models. We evaluated the incidence of AM between 1975 and 2016 in the SEER database. A trend of increasing incidence of AM was observed according to the SEER database. The estimated annual incidence of AM reported previously was 0.3–0.4 per 1 million,32 although the SEER database may not accurately reflect the true incidence of AM because SEER data also have limitations such as bias in registration data and incomplete information. However, this increase in AM according to SEER data may warrant increased clinical attention.
According to the SEER data analysed in this report, the median age at diagnosis was 71 years and women were more predisposed to AM than men. These findings indicate that it is important to take more effective measures to detect and manage this cancer among female patients. In addition, higher age at diagnosis, more advanced stage and LN positivity were strongly associated with worse survival rates.33 34 A total of 795 patients with AM were analysed and age at diagnosis, location, stage, LN positivity and chemotherapy were associated with AM-SS. The American Joint Committee on Cancer classified AM as a local disease with regional nodal involvement and distant metastasis.35 According to our study, patients with distant metastasis had poorer AM-SS and OS than those with local or regional stage distribution. These results support the use of the SEER stage system adopted in the present study. However, this system seems less accurate because other high-risk factors can also contribute to the prognosis of AM. We established a prognostic nomogram for predicting AM-SS using independent prognostic factors on multivariate analysis. The validation cohort, comprising 40 patients with AM enrolled from the eastern part of the country, also showed good agreement with our developed nomogram. The result indicates that the developed nomogram could provide good prognostic function.
Many recent studies have demonstrated that patients with AM show good prognosis with surgery and the results of the present study are consistent with some of these previous studies.36 37 Specially, we conducted PSM analysis to eliminate bias; in other words, our study results may be more accurate than those reported previously. A total of 550 patients for whom complete surgical data were available were enrolled in this study. After the PSM analysis of patients who did or did not undergo surgical treatment, we found that those who underwent surgical treatment achieved significantly better survival benefits than those who did not undergo surgery (OS: log-rank=17.41, p<0.01; AM-SS: log-rank=14.55, p<0.01). Early studies are more likely to recommend aggressive surgery to achieve local oncological radicality. However, recent studies suggest no significant differences between local wide excision and abdominoperineal resection/anterior resection despite the latter significantly reducing local recurrence than the former.38 Another 2010 study of 145 patients with AM concluded that surgery type did not affect the OS or AM-SS of patients enrolled from the SEER database.39 However, the authors of that study did not exclude confounding variables that may have contributed to incorrect prediction of AM prognosis. In this study, we controlled for similar baseline variables using PSM analysis and found no significant difference between LR and ER in terms of OS and AM-SS. This finding indicates that extensive surgery and lymphadenectomy did not improve survival in patients with primary AM.
Our study possesses both benefits and limitations. Although we performed a partial analysis of the incidence of AM in this study, the results were only analysed from a single database and the credibility of data on AM incidence may be reduced due to the long span of the study. In addition, due to the limited information registered for patients with AM in the SEER database and the recording or coverage of the SEER database, we have not found more factors when analysing prognosis-related risk factors. However, it also gives us enlightenment that we need to register more new potentially meaningful risk factors when establishing AM patient information. Finally, due to the rare cases of patients with AM, we actually included all the 40 patients that could be tracked at our institute. Therefore, the C-index was not good enough when we performed external verification of the nomogram.
Herein, we found that the incidence of AM has shown a trend of increasing incidence over the past few decades. The nomogram we developed based on analysis of the SEER database showed good predictive value. Patients with AM could benefit from surgery in terms of better AM-SS and OS; however, extensive surgery and lymphadenectomy may not improve both OS and AM-SS.
Data availability statement
Public data of this study are available from the SEER database (https://seer.cancer.gov). The validation data are from our hospital and available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
XL and LQ contributed equally.
Contributors CZ is responsible for the overall content as the guarantor.XL and LQ conceived the study and wrote the manuscript. YW and YK collected and analysed the data. CH and CZ reviewed the data. PL conceived and revised the manuscript. All authors approved the final draft of the manuscript.
Funding This work was supported by the National Natural Science Foundation of China (81974386) and Central South University Xiangya Clinical Big Data Project (no: 2013-27).
Disclaimer The funding agency had no role in study design; data collection, analysis and interpretation; or writing of the manuscript.
Competing interests None declared.
Ethics statement This study involves human participants and was approved by Xiangya Hospital of Central South University Ethics Committee (no: 202110188).
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.