Article Text
Abstract
Introduction Given the burdens of treatment and poor prognosis, older adults with kidney failure would benefit from improved decision making and palliative care to clarify goals, address symptoms, and reduce unwanted procedures. Best Case/Worst Case (BC/WC) is a communication tool that uses scenario planning to support patients’ decision making. This article describes the protocol for a multisite, cluster randomised trial to test the effect of training nephrologists to use the BC/WC communication tool on patient receipt of palliative care, and quality of life and communication.
Methods and analysis We are enrolling attending nephrologists, at 10 study sites in the USA, who see outpatients with advanced chronic kidney disease considering dialysis. We aim to enrol 320 patients with an estimated glomerular filtration rate of ≤24 mL/min/1.73 m2 who are age 60 and older and have a predicted survival of 18 months or less. Nephrologists will be randomised in a 1:1 ratio to receive training to use the communication tool (intervention) at study initiation or after study completion (wait-list control). Patients in the intervention group will receive care from a nephrologist trained to use the BC/WC communication tool. Patients in the control group will receive usual care. Using chart review and surveys of patients and caregivers, we will test the efficacy of the BC/WC intervention with receipt of palliative care as the primary outcome. Secondary outcomes include intensity of treatment at the end of life, the effect of the intervention on quality of communication (QOC) between nephrologists and patients (using the QOC scale), the change in quality of life (using the Functional Assessment of Chronic Illness Therapy-Palliative Care scale) and receipt of dialysis.
Ethics and dissemination Approvals have been granted by the Institutional Review Board at the University of Wisconsin (ID: 2022-0193), with each study site ceding review to the primary IRB. All nephrologists will be consented and given a copy of the consent form. No patients or caregivers will be recruited or consented until their nephrology provider has chosen to participate in the study. Results will be disseminated via submission for publication in a peer-reviewed journal and at national meetings.
Trial registration number NCT04466865.
- Adult palliative care
- Dialysis
- Chronic renal failure
- Adult nephrology
- MEDICAL ETHICS
- End stage renal failure
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Adult palliative care
- Dialysis
- Chronic renal failure
- Adult nephrology
- MEDICAL ETHICS
- End stage renal failure
STRENGTHS AND LIMITATIONS OF THIS STUDY
The communication intervention under study uses a graphic aid that is not only useful for patients, but also functions to measure adherence to the intervention.
There is a diversity of study sites and utilisation of complementary data from chart review, patient self-report and caregiver report.
There has been difficulty enrolling seriously ill older adults during the COVID-19 pandemic.
Nephrologists will need to use the tool during a busy in-person or virtual clinic visit.
Each patient’s overall health trajectory and time to death will vary, making study of end-of-life outcomes complex.
Introduction
Older adults with advanced kidney disease, particularly those with comorbidities and/or frailty, have life-limiting illness,1–3 which will lead to major changes in quality of life and functional status over time. As their kidney function declines, most will consider dialysis. Yet older adults often initiate dialysis without understanding their prognosis, the investment of time needed for receipt of dialysis and the life-sustaining nature of this treatment. Although some will gain a survival advantage, patients with multiple comorbidities will often fail to achieve this goal.4 For example, median survival after starting dialysis is 15.6 months for patients age 80–85, and 20% will die within 3 months of dialysis initiation.5 Patients who do achieve prolonged survival with dialysis will nonetheless experience treatment burdens and distressing symptoms. These include time spent in dialysis; repeat hospitalisations; vascular access complications; and symptoms of pain, sleep disturbance, depression, itching, oedema, constipation, nausea and loss of appetite.6 When patients choose to forgo dialysis, they experience fewer treatment burdens, but their symptoms are similarly formidable.7 8
Clinicians, focused on disease management, describe dialysis options but struggle to concurrently communicate the severity of disease, the hardship of treatment and overall prognosis. As such, patients are often unaware of how sick they truly are.9 Because dialysis is described as ‘kidney replacement’, it is regarded as a straightforward solution. Describing dialysis as a ‘fix’ for kidney failure does little to reveal how a patient might experience dialysis, or expected downstream outcomes, such as predictable changes in functional status or long-term prognosis.10 In one study, investigators found that for roughly 90% of patients, nephrologists did not disclose prognosis.11 Studies have found that between 20% and 60% of older adults regretted their decision to start dialysis.12 13
Given these concerns, nephrologists might benefit from a stronger framework to discuss dialysis initiation with older adults who have limited survival. This would support discussion of treatment options while describing the experiences and outcomes of treatment within the context of the patient’s overall health trajectory. Because kidney failure is life-limiting, the framework should also provide an entrée to concurrent palliative care, regardless of the patient’s choice about dialysis. Palliative care, with or without dialysis, is recommended for all patients with advanced kidney disease and limited survival to support advance care planning, symptom management and, when needed, high quality end-of-life care.14 15
To address this problem, we developed a communication tool called Best Case/Worst Case (BC/WC) to help nephrologists better describe a treatment choice between life with or without dialysis. The tool uses a strategy called scenario planning and a graphic aid to help patients and their caregivers anticipate and prepare for a future with kidney failure. By using scenario planning—narrative description about plausible futures—nephrologists can translate evidence about the patient’s prognosis within stories to demonstrate a range of plausible futures. We theorise that training nephrologists to use this tool to support shared decision making about dialysis will increase receipt of guideline-recommended concurrent palliative care for older adults with life-limiting kidney failure.16
We designed a multisite, cluster randomised clinical trial to test the effect of training nephrologists to use the BC/WC tool on receipt of palliative care, quality of life and quality of communication for older adults with kidney failure and limited life expectancy. First, we discuss the theoretical foundations of the intervention and study design. We then describe the research protocol including participant characteristics, data collection, outcomes and analysis plan.
Scenario planning
The BC/WC communication tool uses an approach called ‘scenario planning’ to facilitate decision making in the setting of uncertainty. Scenario planning was originally developed in the 1950s for military planning. It was then popularised for broader use by Pierre Wack,17 18 an economist, to translate vast probabilistic information into narrative description to facilitate strategic decisions. Rather than emphasising precise isolated risks, this technique generates multiple plausible futures, prompting decision-makers to visualise what might happen under different sets of assumptions. Scenario planning is distinct from standard medical practice that uses risk prediction and statistics to describe prognosis. Scenario planning enables clinicians to say, “I cannot see the future, but if all goes well, this is what’s likely to follow, and if things go poorly, this is what we can expect.” By highlighting the interaction between forces that drive change and providing an organised way to consider alternative futures, scenario planning promotes insight.6 Although it has been successfully applied to a wide range of decisions in business and government, scenario planning is not broadly used clinically.
We adapted scenario planning for healthcare decision making in the BC/WC communication tool.19 This tool is distinct from decision aids that demonstrate event rates and probabilities, represent outcomes numerically20 or pictorially, and function by activating patients for decision making before meeting with a clinician (ie, with brochures, educational videos, web programmes and decision tables).21 22 In contrast, nephrologists use the BC/WC tool directly with patients to illustrate treatment options, convey a clear message about prognosis and acknowledge uncertainty using language such as, ‘this is what we are hoping for’ or ‘this is what we are worried about.’ By using scenario planning to translate evidence about the patient’s overall health within narratives that show the range of plausible futures, nephrologists can elicit patients’ opinions about specific health states and recommend goal-concordant treatment. Use of this tool can encourage patients to comprehend a new, previously unimaginable reality and prepare for major shifts in a way that simple prognostication (forecasting) cannot. Moreover, scenario planning allows patients to anticipate unwanted events, such as recurrent hospitalisations, which can facilitate use of palliative care consultation to alleviate symptoms and clarify care goals regardless of the patient’s dialysis initiation decision.
How the BC/WC tool works
The clinician verbally describes the ‘BC,’ ‘WC’ and ‘most likely’ stories about the experience of each treatment option while using a graphic aid to help patients follow along and have a record of the conversation (figure 1). Vertical bars represent each treatment option; their length shows the range of outcomes and the magnitude of the difference between the ‘BC’ (star), the ‘WC’ (box) and a ‘most likely case’ (oval). The clinician also writes notes about each option on the diagram. A copy of the graphic aid is stored in the patient’s chart. Patients also retain a copy to discuss options with family and to support future conversations with their nephrologist and other clinicians. This approach—using personalised information and a graphic aid—supports best practices to improve understanding of complex health information, especially for people with low health literacy.23 24
Example of the ‘Best Case/Worst Case’ graphic aid used by nephrologists as part of the communication tool. Vertical bars represent treatment options; their length shows the range of outcomes and the magnitude of the difference between the ‘best case’ (star) the ‘worst case’ (square) and a ‘most likely case’ (oval). Nephrologists write short notes for patients on the diagram while describing possible scenarios that are derived from clinical experience and data. The ‘most likely’ box is intentionally left blank for nephrologists to tailor to the patient.
Study design
This study is designed to test the effect of a decision-making intervention on the quality of care received, based on evidence that patients with life-limiting illness benefit greatly from concurrent palliative care.25 26 We hypothesise that improving conversations about dialysis will help patients receive palliative care consultation and support subsequent treatment decisions that align with personal goals.
Patients who are more informed about the experience of dialysis and their overall health trajectory might take advantage of palliative care earlier in their course of illness. As compared with patients with terminal cancer and heart failure, patients with kidney failure are more than twice as likely to be admitted to the ICU and less than half as likely to be admitted to hospice in the final month of life.27–29 Fewer than 6% of patients on dialysis have had the opportunity to discuss end-of-life wishes.30 Palliative care for patients with poor prognosis is supported by the American Society of Nephrology,14 15 however, barriers to palliative care include (1) patient lack of awareness about the life-limiting nature of kidney failure and (2) an illness trajectory typified by a slow overall decline with interval catastrophic events and partial recovery, not a sharp and obvious deterioration.31 These barriers cannot be overcome by simply referring patients to palliative care.
Outcomes
As there is no ‘best treatment choice’ for these patients as a group, dialysis initiation is not the primary outcome. Measuring change in the rate of dialysis initiation does not reflect whether patients have received care in accordance with their goals. Moreover, there are unyielding challenges in attempting to measure receipt of goal-concordant care. One would first need to assess a patient’s goals and values, and then determine whether the treatment received was consistent with these values. Because clinicians do not typically elicit patients’ goals and values, measurement of this variable could change the outcome of interest.
We also considered assessing decisional conflict and regret but found this measurement to be unsatisfactory. Ascertainment of decisional conflict is hampered by the framing of the clinical question.32 For example, if a nephrologist says, “You need dialysis or you will die,” patients will report little decisional conflict or regret on starting dialysis, yet this outcome does not capture whether patients are well-informed or if they have received goal-concordant care. Aiming to reduce decisional conflict suggests conflict around uncertainty is inherently undesirable, whereas conflict during deliberation might be necessary to make a decision that best reflects a patient’s values.
We theorise that patients whose nephrologists communicate better will receive better care. Therefore, we will measure receipt of palliative care as the primary outcome. Teaching nephrologists to use the BC/WC communication tool will lead to increased receipt of palliative care via multiple pathways: first, via direct provision of information about prognosis and health trajectory, second, through improving the QOC about options and outcomes, and third, via improving decision making about dialysis initiation.
Methods and analysis
Design and setting
To test the efficacy of the BC/WC intervention, we will use parallel randomisation with a wait-list control, randomising nephrologists within each site to receive intervention training at study initiation or on study completion. Patients in the control group will receive usual care; patients in the intervention group will receive care from a nephrologist trained to use the BC/WC communication tool. We anticipate 2 years will be needed to enrol a full cohort of 320 patients. The estimated total number of study participants is 680 which includes 320 patients, up to 320 caregivers and 40 nephrologists. We will follow patients and their caregivers for 2 years via survey administration and chart review. We are conducting the study in outpatient nephrology clinics at ten academic medical centres across the USA (table 1).
Trial study sites and their locations
The study is coordinated by the University of Wisconsin study team with technical support provided by the Palliative Care Research Cooperative Group (PCRC).
Screening and enrolment
Nephrologists
We will enrol nephrologists, including doctors and advanced practice providers, who care for older patients with advanced chronic kidney disease considering chronic dialysis. We will exclude trainees and nephrologists whose practices are focused on other aspects of clinical nephrology. Qualifying nephrologists will be offered US$250 for completing training.
Patients
We plan to enrol 320 (160 per arm) patients with an estimated glomerular filtration rate (eGFR) of less than or equal to 24 mL/min/1.73 m2 who are age 60 years and older. We will enrol patients who have an estimated survival of 18 months or less (with or without dialysis) based on meeting at least one of the three criteria defined by Robins33: age greater than 80, evidence from the medical record that the patient has comorbid illness such that the modified Charlson score34 is 4 or greater, or a ‘no’ response to the ‘surprise Question’ (‘Would you be surprised if this patient died in the next year?’) from the patient’s nephrologist.35 We will exclude patients who (1) are currently treated with dialysis, (2) lack decision-making capacity36 or (3) do not speak English.
Caregivers
We will invite one family member or informally designated ‘like family’ caregiver per patient to participate. Patients can participate without caregivers; however, caregivers cannot participate without a corresponding patient. Caregivers must be at least 18 years old. We will exclude caregivers who don’t speak English, and those who do not have decision-making capacity. Including caregivers will help reduce missing data by providing a proxy measurement when study participants with life-limiting illness are too sick to respond.
Recruitment and enrolment
Patients who meet inclusion criteria will be contacted prior to their visit to obtain consent. Patients will receive US$20 at enrolment and US$10 for each follow-up survey. Caregivers will receive US$15 at enrolment and US$5 for each follow-up survey. Study enrolment commenced on 1 January 2021 with an original estimated primary completion date of January 2025 (2 years of enrolment, 2 years of follow-up).
Randomisation and blinding
Nephrologists will be clustered by site, and are randomised to the control or intervention group in a ratio of 1:1. A block randomisation scheme will be used with blinded block size. Nephrologists will not be blinded to study group and will be informed of the goals of this study. To maintain blinding of local research staff performing data collection, the Wisconsin-based education team will coordinate individual training with nephrologists.
To reduce the possibility of study staff exposure to the nephrologist’s randomisation assignment, we will provide regular reminders to nephrologists that they should not reveal their treatment group to study staff. To decrease ascertainment bias, study staff will adhere to a study script during interactions with nephrologists and during the survey administration with patients and caregivers. Patients and caregivers will be blinded to the measured outcomes of this study.
Intervention
The original version of the intervention was developed with input from older adults via focus groups conducted at senior centres in Wisconsin.37 The intervention was then modified to specifically to fit dialysis decision making with support from patients and their caregivers. In interviews, they expressed enthusiasm for content about prognosis and decision making and ease of use within their clinic visit with the nephrologist.
The intervention comprises a 2-hour one-on-one training session, three additional 20 min coaching sessions, debriefs every 2 months during the first year and quarterly debriefs in the second year. The 2-hour training session includes a 10 min introduction, coach demonstration, individual preparation and practice with standardised patients, and expert feedback, that is, coaching. The primary skill targeted is translation of clinical knowledge and prognostic information into the BC/WC format, using scenario planning and the graphic aid. Nephrologists will receive specific instruction on how to refer patients to palliative care, including scripted language: for example, “I’d like you to see our palliative care team. They can help you feel as well as you can for as long as you can.”
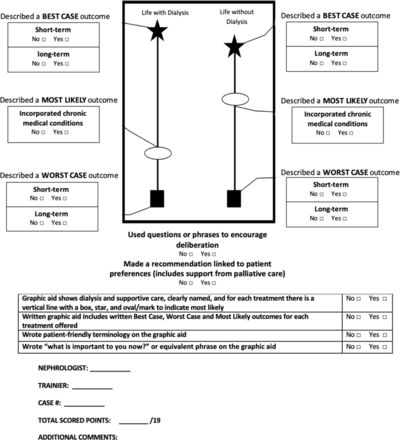
The training programme culminates in an assessment of nephrologists’ use of the tool with a standardised patient. Instructors will assess the fidelity of the nephrologist’s use of the BC/WC tool using a 19-item checklist of adherence criteria (figure 2). Nephrologists who do not achieve minimal competence (<14/19) will receive additional training until they achieve competence.
Best Case/Worst Case skills checklist and observation form. Instructors evaluate 19 criteria to assess nephrologists’ performance after they have completed training. Competence is defined as achieving a score of at least 14/19.
Following the initial training, instructors will contact nephrologists for three additional 20 min coaching sessions. This will occur every 2 weeks for 6 weeks after training when nephrologists have had the opportunity to use this tool clinically. These sessions will focus on troubleshooting in-the-moment issues with additional support for integrating the tool within a busy nephrology clinic. Due to the COVID-19 pandemic, in-person training, standardised patient scenarios and coaching were adapted for virtual education on the Zoom platform.38
To ensure intervention adherence throughout the study, study staff will remind nephrologists to follow the assigned communication condition prior to an enrolled patient’s clinic visit. Study staff will notify all nephrologists each time one of their patients has enrolled in the study. Specifically, study staff will say, ‘This patient is enrolled in the study, if you are in the intervention group, please use the communication tool during this visit.’ To document use of the tool, study staff will provide a folder including a triplicate carbon copy of the graphic aid template (one study copy, one copy for the patient and one copy for the patient’s chart), a written reminder to use the BC/WC tool (as appropriate), and blank carbon copy paper for notes written by study-enrolled nephrologists. Nephrologists in the waitlist control group may use any of the forms as they wish or disregard them. We anticipate minimal contamination between nephrologists as our experience indicates training and support is required to use the tool. Nephrologists will return the folder and its contents sealed in a preaddressed envelope directly to the Wisconsin education team.
To maintain use of the tool over time, the education team will conduct debriefings with nephrologists every 2 months during year one and quarterly debriefings in year two. Instructors will review the submitted graphic aids, provide feedback on fidelity to nephrologists, and take field notes to evaluate training and implementation of the tool.
Data collection
Patient and caregiver surveys
Prior to the patient’s visit with the nephrologist, study staff will administer the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-Pal V.4) survey.39 We obtain this prior to the patient’s first visit on study as patients who receive new information about their prognosis may rate their quality of life lower as a result. Simultaneously, caregivers will complete a single-item literacy screener and the Cambridge Palliative Audit Schedule (CAMPAS-R), which allows us to assess the patient’s quality of life (QOL) as reported by caregivers.
Within 48 hours after the patient’s visit with the nephrologist, study staff will administer the QOC questionnaire via telephone. Unlike other measurements of physician communication that have high ceiling effects and limited ability to measure change, the QOC includes seven items specific to end-of-life communication, which, if not performed by the clinician, are scored as zero. This will allow us to discriminate between QOC attributable to patient satisfaction vs content.
We will repeat the quality-of-life measurement every 3 months for 2 years after enrolment or until patient death. We will administer the Quality of Death and Dying (QODD)40 survey to enrolled caregivers between 30 days and 3 months after patient death for patients who die while on study.
Chart review
We will use monthly chart review to record treatments received, including palliative care consultation, ICU admissions, hospitalisations, emergency room visits, dialysis initiation and termination, life supporting treatments including intubation and CPR, days in hospice and death. These data will be collected and managed using REDCap electronic data capture tools hosted at the University of Wisconsin.41 42 We will ensure data quality through an independent review of all data entry for one patient every 10 patients.
Outcomes
Palliative care
To assess receipt of palliative care, we will use chart review and patient and caregiver report to determine patient receipt of any inpatient or outpatient palliative care consultation within 12 months of enrolment. Palliative care consultation must be clearly marked and provided by a clinician with palliative care training (MD, DO, PA, NP, MSW, RN or Chaplain). The visit must have documented discussion of at least one of the following: advance care planning, symptom management or end-of-life care.
Intensity of treatment
To measure intensity of treatment received at the end of life we will ascertain whether patients have had an ICU admission within 30 days of death and ICU admission, emergency room (ER) visit, or hospital admission within 30 days of death as a composite outcome.
Secondary outcomes
Additional secondary outcome measures include health-related quality of life, QOC, dialysis initiation, death on study, caregiver bereavement and quality of life and QOC as assessed by caregivers (table 2).
Primary and secondary outcomes
Planned analysis
Sample size calculation
The sample size estimate, 320 patients (160/group), is based on the primary hypothesis that patients in the intervention arm will be more likely to receive palliative care. This study is powered to detect a 10%–15% absolute difference in the care patients receive, consistent with other interventions designed to effectively increase access to palliative care.43–46 Smaller differences are unlikely to be considered meaningful to clinicians, patients or researchers.26 We desire a two-sided type I error rate of 0.05 for each aim and estimate that the between-physician variance is around 10%. We plan to use fixed effects to account for clustering by site because it is faithful to our study design and controls for confounding related to imperfect randomisation due to site imbalances better than a random effects model.
Primary outcomes analysis
Our primary analysis will compare the efficacy of the BC/WC training programme relative to usual care in regard to receipt of palliative care consult within 12 months of study enrolment. We will use summary statistics to describe, by group, patient and nephrologist characteristics. As patient comorbidity and baseline functional status are highly predictive of outcomes, we will adjust for these covariates to avoid spurious results, and to increase power by reducing residual error in the response. We will use an intention-to-treat analysis and compare outcomes between the two arms using a proportional odds cumulative incidence model, where death is incorporated as a competing risk. The intervention will be a binary predictor and the treating physician will be a random effect to account for the correlation within the physician. Site will be included as a fixed effect.
Secondary outcomes analysis
We will conduct secondary analyses with intervention as the main predictor while adjusting for demographic and clinical factors. All analytical methods used will account for the cluster effect of the treating physician by incorporating a random-intercept for physician. We will analyse the cumulative receipt of palliative care throughout the length of follow-up using a proportional odds cumulative incidence model.47 Death will be treated as a competing risk. Regarding quality of life, we will conduct a linear mixed effects regression on the difference in FACIT-Pal scale between baseline and death or at 2 years after enrolment, whichever occurs first. We will use a linear mixed-effects model with the QOC scale as the outcome.
We will perform additional analyses to test and quantify receipt of dialysis, and caregiver outcomes. We will analyse receipt of dialysis using the proportional odds cumulative-incidence model for competing risks with death as a competing risk. We will analyse the time from enrolment to death using a mixed-effects Cox regression. We will use linear mixed-effects model to analyse caregiver outcomes: QODD (for caregivers of decedents only), CAMPAS-R and QOC (family member version). We will use qualitative content analysis to analyse field notes from nephrologist training and follow-up sessions.
Missing data
Study participants are subject to attrition due to death (ADD), attrition due to illness (ADI) and attrition at random. ADD and ADI can lead to non-ignorable missing data because the missing mechanism is related to the patient’s health status, which is potentially an effect of the intervention. We expect that missing outcomes will be minimal for receipt of palliative care, intensity of treatment and QOC because they are measured at early stages (within 12 months) or 30 days retrospectively from death. The QOL measure, however, is likely to suffer from both ADD and ADI. We plan to compare the difference between QOL at baseline and death or 2 years after enrolment (whichever comes first) to handle the fact that patients will have a different number of observations. Similar strategies have been used by other studies of palliative care interventions reflecting a quality of life/length of life trade-off.25 Receipt of palliative care and dialysis are subject to ADD. Death will be incorporated as a competing risk to account for different lengths of follow-up in our secondary analysis. Because death is not likely to be independent of the outcomes, we will conduct analyses using inverse probability censoring weighting48 adjusting for confounders such as patient QOL measures as sensitivity analysis.
Protocol modifications
Few patients died during the first 12 months of data collection, which was inconsistent with their estimated survival based on risk prediction. This observation led us to believe that it would be unlikely to have enough observed death on study to evaluate the effect of the intervention on care received at the end of life. With permission from the funder and the data and safety monitoring board (DSMB), we reclassified the outcome ‘intensity of treatment at the end of life’ from a coprimary outcome to a secondary outcome. In addition, to address the COVID-19-pandemic-related decrease in enrolment we modified the approved proposal to expand enrolment criteria to include patients with eGFR of 24 or less and two additional study sites (as reported herein). These modifications were approved by the funder and the DSMB.
Patient and public involvement
None.
Ethics and dissemination
Ethical review
The aims of the study meet criteria for minimal risk. All participants will provide informed consent and may withdraw from the study at any time without affecting the medical care they receive. Consent forms for patients, caregivers and nephrologists are provided as online supplemental files 1–3, respectively. For nephrologists, study participation will not affect their professional standing. Institutional review board (IRB) approval has been granted at UW (ID: 2022-0193) with study sites ceding review to the primary IRB. We will follow accepted adverse event monitoring procedures including review every 6 months by the data monitoring committee.
Supplemental material
Supplemental material
Supplemental material
Relevance and dissemination
There is currently no level one data supporting the use of scenario planning for patients with life-limiting illness. Study results will be published in peer-reviewed journals and intervention training materials, including the graphic aid, instructional videos and learner manual, are available free of charge at https://patientpreferences.org/bcwc-nephrology/. Durable improvements in serious illness communication will require training in scenario planning, which could be disseminated by stakeholder groups including the American Society of Nephrology and the National Kidney Foundation. Empirical evidence of efficacy would support incentives from payors by rewarding nephrologists for use of the tool. We will distribute final study results to patient, caregiver and nephrologist participants via a study website, reminding them of the website at the time of final data collection. In addition, we will remind participants that study results are publicly available at ClinicalTrials.gov.
By conducting this study in a patient population with one specific decision about initiation of life-sustaining treatment, we can consider how scenario planning might be effective in other settings where treatment decisions vary widely, for example, acute surgical interventions or cancer care. If effective, the BC/WC intervention is easily scalable. This communication tool can be rapidly adapted for other clinicians in order to provide better care for older adults with life-limiting illnesses.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank the Research Coordinators for their dedication to study enrolment, data collection and patient care.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @AmyBZelenski
Contributors MS is the principal investigator for this study. She developed the original study design and protocol together with KLK, PR, BMH, LS, TCC, RJ who provided input on study design, and the site principal investigators ADB, KC, DC, RF, HK, DL, AM, MR, DFW and JY. CB and RK provided study design and technical support. AB is the study manager and has the primary responsibility of coordinating development of all study materials. KH drafted this manuscript and along with AZ helped with development of educational materials. All authors reviewed and approved this manuscript.
Funding This work is supported by the National Institutes of Health (R01AG065365) and by the National Institute of Nursing Research of the National Institutes of Health (NIH) under award number U2CNR014637. KH is supported by a Training Award (T32HL110853) from the NIH and the Resident Research Scholarship through the American College of Surgeons (ACS).
Disclaimer This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the views of the PCRC or ACS.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.