Article Text
Abstract
Objectives Hand, foot, and mouth disease (HFMD) is a viral infectious disease that poses a substantial threat in the Asia-Pacific region. It is widely reported that meteorological factors are associated with HFMD. However, the relationships between air pollutants and HFMD are still controversial. In addition, the interactive effects between meteorological factors and air pollutants on HFMD remain unknown. To fill this research gap, we conducted a time-series study.
Design A time-series study.
Setting and participants Daily cases of HFMD as well as meteorological and air pollution data were collected in Chengdu from 2011 to 2017. A total of 184 610 HFMD cases under the age of 15 were included in our study.
Outcome measures Distributed lag nonlinear models were used to investigate the relationships between HFMD and environmental factors, including mean temperature, relative humidity, SO2, NO2, and PM10. Then, the relative excess risk due to interaction (RERI) and the proportion attributable to interaction were calculated to quantitatively evaluate the interactions between meteorological factors and air pollutants on HFMD. Bivariate response surface models were used to visually display the interactive effects.
Results The cumulative exposure–response curves of SO2 and NO2 were inverted ‘V’-shaped and ‘M’-shaped, respectively, and the risk of HFMD gradually decreased with increasing PM10 concentrations. We found that there were synergistic interactions between mean temperature and SO2, relative humidity and SO2, as well as relative humidity and PM10 on HFMD, with individual RERIs of 0.334 (95% CI 0.119 to 0.548), 0.428 (95% CI 0.214 to 0.642) and 0.501 (95% CI 0.262 to 0.741), respectively, indicating that the effects of SO2 and PM10 on HFMD were stronger under high temperature (>17.3°C) or high humidity (>80.0%) conditions.
Conclusions There were interactive effects between meteorological factors and air pollutants on HFMD. Our findings could provide guidance for targeted and timely preventive and control measures for HFMD.
- infectious diseases
- epidemiology
- public health
Data availability statement
Data may be obtained from a third party and are not publicly available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first study to explore the potential interactive effects between meteorological factors (mean temperature and relative humidity) and air pollutants (SO2, NO2 and PM10) on hand, foot, and mouth disease (HFMD).
The interactive effects between meteorological factors and air pollutants on HFMD were quantitatively evaluated using relative excess risk due to interaction and proportion attributable to interaction.
The daily data of environmental factors were obtained from fixed monitoring stations and were not representative of individual exposure levels.
This study was essentially an ecological study and thus could not confirm causal relationships between environmental factors and HFMD.
Introduction
Hand, foot, and mouth disease (HFMD), a substantial public health issue mainly in the Asia-Pacific region, is a common infectious disease caused by various enteroviruses, with enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) being the major pathogens.1 2 Although HFMD is a self-limiting disease, a portion of patients develop severe and even fatal complications involving neurological or cardiovascular systems.3 Since 1997, multiple outbreaks of HFMD have occurred in the Asia-Pacific region, imposing a substantial disease burden on society.3–5 In China, HFMD has been included as a notifiable infectious disease since May 2008.6 From 2009 to 2018, the annual number of reported cases and deaths of HFMD consistently ranked highest among category C notifiable infectious diseases.7 However, there is no specific treatment, only symptomatic therapy, for HFMD. The EV71 monovalent inactivated vaccine, licensed in China in 2015, protects against HFMD caused by EV71 but not other serotypes of enteroviruses.8–10 Therefore, it is vital to deepen epidemiological knowledge about HFMD and implement targeted and timely prevention and control measures to reduce the burden of HFMD. Currently, extensive studies are exploring relevant environmental factors associated with HFMD, among which meteorological factors and air pollutants are generating widespread concern.11–15
Numerous studies have confirmed that temperature and humidity are two of the most important meteorological factors affecting HFMD, with associations showing nonlinear and lagged effects.11–14 However, to date, studies on the relationships between air pollutants and HFMD are insufficient, with inconsistent results, and mainly focus on lightly polluted areas.16–18 Located in southwestern China, Chengdu bears a double burden of severe air pollution and a high incidence of HFMD due to its unique basin terrain and climate conditions coupled with extensive migration and a very large resident population.19–21 Consequently, it is necessary to explore the associations between air pollutant concentrations and HFMD in Chengdu.
Although much attention has been given to the associations between environmental factors and HFMD, few studies have focused on the effects of the interactions among environmental factors on HFMD. Some research has suggested that investigating the individual effects of meteorological factors or air pollutants alone while ignoring the combined effects of such factors may incorrectly estimate the effects of environmental factors on health outcomes.22–24 Nonetheless, previous studies analysing the effects of the interactions among environmental factors on health outcomes have predominantly concentrated on chronic non-communicable diseases24–29; for example, cardiovascular and respiratory diseases, with few studies on communicable diseases. Hence, it is necessary to explore the effects of the interactions between meteorological factors and air pollutants on HFMD to strengthen the understanding of the relationships between environmental factors and HFMD.
To aid in strengthening the understanding of the influence of environmental factors on HFMD and providing scientific evidence for the development of targeted and timely preventive and control measures for HFMD, this study assessed the associations between environmental factors and HFMD in Chengdu and then investigated the effects of interactions between meteorological factors and air pollutants on HFMD. Further subgroup analyses by sex, age and season groups were conducted.
Materials and methods
Data collection
Located in the western part of the Sichuan Basin in southwest China, Chengdu is the capital city of Sichuan Province, lying between 102°54'−104 °53'E and 30 °05'−31 °26'N and covering a land area of 14 335 square kilometres, with a humid subtropical monsoonal climate.19 By the end of 2017, Chengdu had a permanent resident population of over 16 million and achieved a gross domestic product (GDP) of 195 billion dollars and a GDP per capita of 12.2 thousand dollars, with an urbanisation rate reaching 71.85%.30
The daily number of reported cases of HFMD in Chengdu from 1 January 2011 to 31 December 2017, along with meteorological and air pollution data, were collected. HFMD surveillance data were obtained from the China Information System for Disease Control and Prevention.31 Previous research has shown that approximately 99% of reported cases of HFMD occur in children younger than 15 years old,32 so we included only HFMD cases in those under 15 years old in the analysis. The monitoring data of meteorological factors were acquired from the China Meteorological Data Service Center33 and included mean temperature (°C), relative humidity (%), wind speed (m/s), sunshine duration (hours), air pressure (hPa) and rainfall (mm). Air pollution information, including SO2 (μg/m3), NO2 (μg/m3) and PM10 (μg/m3) concentrations, was obtained from the Department of Ecology and Environment of Sichuan Province.34
Statistical analysis
In brief, we used a distributed lag nonlinear model (DLNM),35 36 which can simultaneously measure both the nonlinear exposure–response association and the lagged effect of the exposure factor, to characterise the associations between different variables and HFMD. The relative excess risk due to interaction (RERI) and the proportion attributable to interaction (API) were then used to quantitatively evaluate the effects of interactions between meteorological factors and air pollutants on HFMD.29 37 38 Finally, we visually displayed the interactions based on the bivariate response surface model.
Distributed lag nonlinear model
A quasi-Poisson distribution was employed to describe the overdispersion feature of HFMD cases.13 The DLNM used to depict the associations between environmental variables and HFMD was structured as follows:
 (1)
(1)
 (2)
(2)
where  represents the daily number of reported cases of HFMD on day t;
represents the daily number of reported cases of HFMD on day t;  is the intercept;
is the intercept;  represents the variable on day t, including mean temperature, relative humidity, SO2, NO2 and PM10;
represents the variable on day t, including mean temperature, relative humidity, SO2, NO2 and PM10;  indicates the cross-basis function of the corresponding variable, for which natural cubic spline functions (
indicates the cross-basis function of the corresponding variable, for which natural cubic spline functions ( ) with various df
) with various df  ) were used to describe the exposure–response and lag-response association of each variable and HFMD, respectively, and 0–14 days was chosen as the lag range.
) were used to describe the exposure–response and lag-response association of each variable and HFMD, respectively, and 0–14 days was chosen as the lag range.  represents the confounders to be adjusted.
represents the confounders to be adjusted.  with 8
with 8 per year was employed to control the long-term trends and seasonal fluctuations.
per year was employed to control the long-term trends and seasonal fluctuations.  and
and  are indicator variables representing the day of the week and a holiday or not, respectively. Finally, the autoregressive term was set to incorporate lags of 1 and 2 days on a logarithmic scale.
are indicator variables representing the day of the week and a holiday or not, respectively. Finally, the autoregressive term was set to incorporate lags of 1 and 2 days on a logarithmic scale.
Interactions
To quantitatively evaluate the effects of the interactions between meteorological factors and air pollutants on HFMD, the median was used as the cut-off point to classify each variable dichotomously.39 40 Then, we constructed the following quasi-Poisson regression model:
 (3)
(3)
where  is the intercept; M and P denote meteorological factors and air pollutants, respectively, both of which are dichotomous variables; and
is the intercept; M and P denote meteorological factors and air pollutants, respectively, both of which are dichotomous variables; and  is the interaction term. The relative risks (RRs) associated with M, P and
is the interaction term. The relative risks (RRs) associated with M, P and  were obtained from Equation (3), namely,
were obtained from Equation (3), namely,  ,
,  and
and 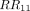 , respectively. The additive interaction between two variables was assessed using the RERI and API,24 29 37 which were calculated as follows41:
, respectively. The additive interaction between two variables was assessed using the RERI and API,24 29 37 which were calculated as follows41:
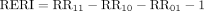 (4)
(4)
 (5)
(5)
When the RERI and API were both equal to 0, there was no interaction between the two tested variables. When the RERI and API were both less than or greater than 0, they indicated an antagonistic or synergistic interaction, respectively.
To further illustrate the effects of the interactions between meteorological factors and air pollutants on HFMD, bivariate response surface models were developed to continuously and visually demonstrate the joint effects of two variables on HFMD.42
Subgroup analyses
Previous studies have suggested that the effects of environmental factors on HFMD vary across sex, age and season groups.16 43–46 Therefore, subgroup analyses were carried out according to sex, age (age<1, 1≤age<3, 3≤age<6, 6≤age<15), and season (May to October and November to April)46 to identify potentially vulnerable populations.
Sensitivity analysis
Sensitivity analysis based on the quasi Akaike information criterion was conducted to determine the key parameters of the DLNMs, including (1) the  of the
of the  controlling the long-term trends; (2) the constraint forms of
controlling the long-term trends; (2) the constraint forms of  ; (3) the lag range; (4) the inclusion form of the
; (3) the lag range; (4) the inclusion form of the  ; and (5) the constraint forms of the exposure–response and lag-response associations of each variable with HFMD.
; and (5) the constraint forms of the exposure–response and lag-response associations of each variable with HFMD.
With reference to most previous studies,25 39 47 we chose the median of each variable as the cut-off point to divide the variables into binary variables. However, the choice of a cut-off point is still controversial. For instance, other studies chose different percentiles, means or turning points of the cumulative exposure–response curves as the cut-off points. The choice of the cut-off point may impact the results. Hence, we further performed sensitivity analysis for various cut-off points.
The ‘dlnm’, ‘splines’ and ‘mgcv’ packages in R software (V.3.6.2) were used to conduct all the analyses.
Patient and public involvement
Patients were not involved in the study design, conduct and development of our research.
Results
Descriptive statistics
A total of 184 610 HFMD cases in patients under the age of 15 were reported in Chengdu from 2011 to 2017. There were more cases in males than in females, with a sex ratio of 1.424. Among the age subgroups, the 1≤age<3 group had the highest proportion of cases (55.810%), while the 6≤age<15 group had the lowest proportion of cases (2.961%). A detailed summary of the variables is provided in table 1. Spearman’s rank correlation coefficients between HFMD cases and environmental factors are shown in online supplemental table S1.
Supplemental material
Descriptive analysis of daily hand, foot, and mouth disease (HFMD) cases, meteorological factors and air pollutants in Chengdu, 2011–2017
Figure 1 displays the time-series trends of the different variables. There were two peaks of HFMD in Chengdu annually, corresponding to April–June and October–December. Mean temperature and relative humidity exhibited clear seasonal characteristics, and the concentrations of SO2, NO2 and PM10 were all higher in winter than in summer.
Daily trends of the number of hand, foot, and mouth disease cases, meteorological factors and air pollutants in Chengdu, 2011–2017.
Associations between environmental factors and HFMD
Figure 2 presents the cumulative exposure–response associations between the different variables and HFMD. With the increase in SO2 concentration, the RR of HFMD first increased, with a peak at 16.6 µg/m3 (RR=1.001, 95% CI 0.992 to 1.009), and then decreased. The cumulative exposure–response curve of NO2 and HFMD was approximately ‘M’-shaped, with two peaks corresponding to 37.9 µg/m3 (RR=1.080, 95% CI 1.004 to 1.161) and 75.9 µg/m3 (RR=1.076, 95% CI 0.992 to 1.167). The RR of HFMD gradually decreased and then levelled off with the increase in PM10 concentration. The cumulative association of mean temperature with HFMD exhibited an inverted ‘V’-shaped curve, with the RR reaching a minimum at −1.8°C (RR=0.745, 95% CI 0.567 to 0.979) and a maximum at 24.1°C (RR=1.008, 95% CI 0.920 to 1.105). The relationship between relative humidity and HFMD was approximately linear. For all five variables mentioned above, the 95% CIs of the curves did not all contain 1, indicating that these variables were statistically significantly associated with HFMD. The 3D plots and contour plots shown in online supplemental figures S1 and S2 also revealed that the exposure–response associations between different variables and HFMD were nonlinear, with lagged effects.
The cumulative effects of different variables on the number of hand, foot, and mouth disease cases in Chengdu, 2011–2017, with the median of each variable as a reference. (A) SO2; (B) NO2; (C) PM10; (D) mean temperature; (E) relative humidity.
Effects of the interactions between meteorological factors and air pollutants on HFMD
Table 2 shows the interactions between meteorological factors and air pollutants on HFMD, suggesting that there were synergistic effects between mean temperature and SO2 concentration, relative humidity and SO2 concentration, as well as relative humidity and PM10 concentration on HFMD, with individual RERIs of 0.334 (95% CI 0.119 to 0.548), 0.428 (95% CI 0.214 to 0.642) and 0.501 (95% CI 0.262 to 0.741) and APIs of 0.299 (95% CI 0.136 to 0.462), 0.368 (95% CI 0.220 to 0.515) and 0.377 (95% CI 0.234 to 0.521), respectively. The effects of the interactions of the other pairs of variables on HFMD were not statistically significant.
Analysis of the interactive effects between meteorological factors and air pollutants on hand, foot, and mouth disease in Chengdu, 2011–2017
Figure 3 shows the joint effects of meteorological factors and air pollutants on HFMD. The effects of air pollutants on HFMD varied across different levels of meteorological factors, which means that there may be interactions between meteorological factors and air pollutants on HFMD.
Bivariate response surfaces of mean temperature and air pollutants (A–C) and relative humidity and air pollutants (D–F) on the number of hand, foot, and mouth disease (HFMD) cases in Chengdu, 2011–2017. The colour gradients from red to yellow show the fitted value, that is, the logarithmic value of the number of HFMD cases, from least to most.
Subgroup analyses
Online supplemental table S2 illustrates the effects of the interactions between meteorological factors and air pollutants on HFMD by sex. Consistent with table 2, the results in online supplemental table S2 suggest synergistic effects between mean temperature and SO2 concentration, relative humidity and SO2 concentration, and relative humidity and PM10 concentration on HFMD in both sex subgroups, with higher risks in females.
Online supplemental table S3 reveals the effects of the interactions between meteorological factors and air pollutants on HFMD by age. However, we did not obtain consistent conclusions about the effects of the interactions on HFMD in all age groups. Specifically, there was a synergistic effect of the interaction between mean temperature and SO2 concentration on HFMD in all age groups except for the 6≤age<15 group, and the risk was highest in the 1≤age<3 group. There were synergistic effects of the interactions between relative humidity and SO2 concentration as well as relative humidity and PM10 concentration on HFMD in all age groups, and the risks were highest in the 6≤age<15 group. In addition, we found antagonistic effects of the interaction between mean temperature and NO2 concentration on HFMD in both the age<1 and 6≤age<15 groups as well as mean temperature and PM10 concentration in the 6≤age<15 group and a synergistic effect of the interaction between mean temperature and PM10 concentration on HFMD in the 1≤age<3 group.
Online supplemental table S4 exhibits the effects of the interactions between meteorological factors and air pollutants on HFMD by season. Similar to table 2, online supplemental table S4 suggests synergistic effects between mean temperature and SO2 concentration, and relative humidity and SO2 concentration on HFMD in the warm season. However, we did not observe the same results in the cold season. Besides, we found an antagonistic effect of the interaction between mean temperature and NO2 concentration on HFMD in the cold season.
Sensitivity analysis
The results of the sensitivity analysis of the key parameters of the DLNMs are shown in the supplementary materials (online supplemental text 1, online supplemental figures S3–13, online supplemental tables S5 and S6). Different cut-off points, including the mean, P75 and the turning point of the cumulative exposure–response curve, were chosen for the sensitivity analysis. The results are presented in the supplementary materials (online supplemental tables S7–S9) and suggest that the interactions were robust considering the choice of various cut-off points.
Discussion
This study investigated the individual and interactive effects of meteorological factors and air pollutants on HFMD. To our knowledge, this is the first study to explore the effects of potential interactions between meteorological factors (mean temperature and relative humidity) and air pollutants (SO2, NO2 and PM10) on HFMD. The results indicated that environmental factors are associated with HFMD. In addition, we found synergistic effects of interactions between meteorological factors and air pollutants on HFMD, which varied across sex, age and season subgroups.
We observed that the association between SO2 concentration and HFMD produced an inverted ‘V’-shaped curve with the RR peaking at 16.6 μg/m3, which was consistent with two studies conducted in Shijiazhuang and Tianjin.48 49 Originating from numerous industrial processes, SO2 has been confirmed to affect human health by irritating the respiratory tract, promoting oxidative damage and decreasing immunity.50–53 However, Wei et al showed that SO2 exposure was associated with an increased risk of HFMD in Hefei,46 and Yan et al found no relationship between SO2 exposure and HFMD in Shenzhen.16 Additional evidence is needed to confirm the association between SO2 and HFMD.
An ‘M’-shaped relationship between NO2 concentration and HFMD was revealed in our study. Similarly, both Liu et al and Peng et al revealed that low concentrations of NO2 showed protective effects against HFMD, while high concentrations of NO2 induced harmful effects.48 54 NO2, which is mainly derived from vehicle emissions, is closely related to elevated biomarkers of systemic inflammation and tissue repair.55 56 However, Yan et al noticed an increased risk of HFMD with increasing NO2 concentrations in only children under 1 year of age in Shenzhen,16 and Gu et al showed that the NO2 concentration was approximately linearly and positively correlated with HFMD in Ningbo.17 Inconsistencies among various studies are possibly due to different pollution levels of NO2. Until September 2021, Chengdu was second only to Beijing, China, with regard to the number of motor vehicles. The average concentrations of NO2 during the study period in Shenzhen and Ningbo were 42.3 μg/m3 and 15.5 μg/m3, respectively, which were both lower than 53.4 μg/m3, as reported in Chengdu. Additional studies on the relationship between NO2 and HFMD are needed.
We found that PM10 concentration was negatively correlated with the risk of HFMD, similar to the results of several studies conducted in different regions in China.16 54 57 For PM10, evidence from previous research demonstrated possible mechanisms by which PM10 affects HFMD, including promoting adhesion of enteroviruses,58 facilitating the spread of enteroviruses,58 59 and reducing the immunity of the host.59 60 Nonetheless, Huang et al discovered a positive association between PM10 concentration and HFMD in only female children,61 while a study conducted in Ningbo demonstrated that PM10 was not statistically associated with HFMD.18 In Chengdu, the daily average concentration of PM10 from 2011 to 2017 was 112.1 μg/m³, exceeding the WHO recommended standard by more than twofold.62 Different pollution levels of PM10 may contribute to the differences in the results of various cities.
Furthermore, this study indicated that SO2, NO2 and PM10 concentrations all had protective effects against HFMD at high concentrations, which can be explained by the increased health consciousness of the public. People voluntarily adopt a series of protective measures to reduce their exposure to air pollutants when the air quality is poor, for instance, by wearing masks, reducing their time outdoors, using air purifiers and maintaining good hygiene practices, thus reducing the risk of HFMD. Currently, research on the associations between air pollutants and HFMD is quite limited, and more research is needed in various regions with different pollution levels.
In addition, we found an inverted ‘V’-shaped relationship and an approximately positive linear association of mean temperature with HFMD and of relative humidity with HFMD, similar to previous research.14 44 Meteorological factors affect HFMD by influencing enterovirus infectivity,37 63 human immunity64 and human activities.65
The results of the interactions suggested that there were synergistic effects of the interactions between mean temperature and SO2 concentration, relative humidity and SO2 concentration, as well as relative humidity and PM10 concentration on HFMD, indicating that the effects of SO2 and PM10 concentrations on HFMD were stronger under high temperature (>17.3°C) and high humidity (>80.0%) conditions. In accordance with most previous studies exploring the effects of interactions on various health outcomes,25 66–68 our study demonstrated synergistic effects of interactions between meteorological factors and air pollutants on HFMD. However, research on the effects of interactions between environmental factors on HFMD is very limited. Only a study conducted in Guangdong showed that there were interactive effects of air quality index (AQI) and temperature as well as AQI and relative humidity on HFMD.37 No experimental studies have explored the exact mechanisms of the effects of interactions between environmental factors on HFMD. Previous studies have suggested that different levels of temperature or relative humidity may change the effects of air pollutants on humans via various mechanisms.29 In a cold environment, the respiratory mucus cilia experience a functionality reduction, which may affect the human body’s ability to clear air pollutants.69 Similarly, changes in relative humidity in the external environment may affect the lung function and airway resistance of humans, which further influence the body’s ability to resist air pollutants.70 Based on the routes of transmission of enteroviruses, we speculated the potential reasons why SO2 and PM10 concentrations were linked with a higher risk of HFMD under high mean temperature (>17.3°C) and high relative humidity (>80.0%) conditions as follows: (1) pathogens are more likely to survive in the external environment under suitable temperature and humidity conditions.71 When the ambient humidity is relatively high, enteroviruses are more prone to adhere to the surface of particulate matter or environmental objects, which increases the possibility of transmission. (2) Temperature can affect the dispersion of air pollutants by influencing the activity of atmospheric convection. Activity will be enhanced when the temperature is high, thus facilitating the transmission of enteroviruses.71–73 (3) Exposure to air pollutants can lower immunity in humans.71 Moreover, uncomfortable temperature and humidity conditions can cause a range of physiological responses, such as increased sweating and respiratory rate.74 Both of these responses make humans more susceptible to pathogens. Further studies are urgently needed to explore the effects of potential interactions and the underlying mechanisms of environmental factors on HFMD.
In the sex subgroup analysis, we found that females were more susceptible to HFMD than males. The different sex-specific associations may be due to different immune responses, physiological functions and exposure opportunities.75–78 We did not obtain a clear conclusion regarding the interaction effects in the age subgroups. Nevertheless, the results of the interactions suggested that children in the 1≤age<3 and 6≤age<15 groups were more vulnerable than those in the other age groups. Children aged 1–3 years were more vulnerable than their counterparts, perhaps because the antibodies obtained from their mothers wear off quickly and their immune systems are still immature.79 In addition, children in the 1≤age<3 age bracket mostly attend kindergarten or childcare institutions where they are in contact with many susceptible children, which increases the risk of HFMD. Compared with the others, children in the 6≤age<15 bracket are predominantly school-aged children, and they are more vulnerable as they have greater exposure to outdoor air pollutants.80 During hot and humid conditions, more attention should be given to females and children aged 1–3 and 6–15 years. We found synergistic interactions between meteorological factors and air pollutants on HFMD during the warm season, but not in the cold season. The results of the seasonal subgroup analysis further indicate the possibility of the interactions between meteorological factors and air pollutants on HFMD. Currently, few studies have analysed the seasonal differences in the interactions between meteorological factors and air pollutants on HFMD, which requires more studies to reveal the potential reasons.
This study explored the individual and interactive effects of meteorological factors and air pollutants on HFMD. However, there are still some limitations of this study that must be noted. First, the daily concentrations of air pollutants in Chengdu were obtained from fixed monitoring stations rather than measurements of individual exposure levels, which may lead to errors in the exposure measurements of air pollutants. Second, this study was essentially an ecological study and thus could not confirm causal relationships between environmental factors and HFMD.
Conclusions
This study revealed the comprehensive effects of environmental factors on HFMD in a heavily polluted city located in Southwest China. It can provide some clues for future studies to comprehensively explore the associations between environmental factors and HFMD. Furthermore, our study can help guide the allocation of health resources to susceptible populations and provide guidance for targeted and timely preventive and control measures for HFMD considering environmental factors.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We thank Yue Ma, Fei Yin and Tao Zhang for providing suggestions and comments for this study. We thank the China Center for Disease Control and Prevention for providing the data of notified hand, foot and mouth disease cases.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
JH and YM contributed equally.
Contributors JH participated in the study design, performed the data analyses and interpretation, and drafted the manuscript. YM, TZ and FY participated in the study design and interpretation of the results and helped to finalise the manuscript. QL, YL and TS helped with the data curation. All authors have read and approved the contents of the final version. FY is acting as the guarantor.
Funding This work was supported by the National Natural Science Foundation of China (Grant No. 81872713; Grant No. 81803332), the Sichuan Science and Technology Program (Grant No. 2021YFS0181), and the Chongqing Science and Technology Program (Grant No. cstc2020jscx-cylhX0003).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.





