Article Text
Abstract
Introduction The use of patient-reported outcome measures (PROMs) in clinical practice has the potential to promote person-centred care and improve patients’ health-related quality of life. We aimed to develop an intervention centred around electronic PROMs (ePROMs) for systematic follow-up in patients diagnosed with breast cancer and to evaluate its feasibility.
Methods and analysis We developed a nurse-oriented and surgeon-oriented intervention in PROMs, including (1) an education programme for nurses and surgeons; (2) administration of BREAST-Q as proactive ePROMs during follow-up in patients diagnosed with breast cancer and (3) feedback to nurses and surgeons on PROM scores and a guidance manual for healthcare practitioners. Subsequently, we designed a non-controlled feasibility evaluation on the outcomes acceptability, demand, implementation, practicality and integration. The feasibility evaluation includes qualitative ethnographic studies exploring the user perspectives of patients, nurses and surgeons and quantitative studies to explore the characteristics of the patient population regarding demographic background, response rates and response patterns. The feasibility study was initiated in September 2021, will continue until 2024 and will include approximately 900 patients. EPROMs are collected at the following assessment time points: baseline (after diagnosis, before surgery), 1-year follow-up and 3-year endpoint.
Ethics and dissemination The study will be conducted according to the General Data Protection Regulation and the fifth version of the Helsinki Declaration. The National Committee on Health Research Ethics approved the study according to the law of the Committee § 1, part 4. All data will be anonymised before its publication. The results of the feasibility study will be published in peer-reviewed, international journals.
- Breast surgery
- Breast tumours
- Quality in health care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Patient-reported outcome measures may support a systematic approach for improved person-centred care, including targeted, individual, psychosocial support and assessment of candidates for reconstructive and/or corrective breast surgical therapy.
This study will generate detailed information on the feasibility aspects of the ePROM intervention for person-centred follow-up in women diagnosed with breast cancer.
This multimethod study will result in both detailed, contextualised insights of qualitative data and the generalisable, externally valid insights of quantitative data.
To our knowledge, this is the first study to investigate the proactive use of BREAST-Q as ePROMs in clinical practice for women diagnosed with breast cancer undergoing different types of reconstructive breast cancer surgeries.
Introduction
Breast cancer is one of the most common cancers, with 2.3 million women globally diagnosed with breast cancer in 2020.1 The survival rate of these women is 75% in most developed countries2 and 90% in Denmark.3 In Denmark, national screening programmes and improvements in breast cancer treatment have high priority in the healthcare system, and patients have several options for treatment.4 The treatment of breast cancer is complex, multidisciplinary and refers to standardised national guidelines by the Danish Breast Cancer Group to guarantee the highest standard of treatment and care.5
Standard treatments in the curative setting of breast cancer include surgery and medical treatment as key components. The spectrum of surgical approaches for treating breast tumours includes breast-conserving therapy with or without the use of oncoplastic techniques, or mastectomy alone, or with primary or delayed reconstruction. Treatments may further include chemotherapy, radiation therapy and hormone therapy.6–8 Furthermore, follow-up with reconstructive corrective plastic surgery may support an increased self-rated quality of life.9 10 Irrespective of the treatment intensity, patients are often long-term impaired by multiple side effects, including fatigue, sleep problems, pain, reduced mobility in the shoulder and lymphedema in the arm.11 Psychosocially, breast loss or changes in the appearance of the breast influence individual patients12 who may experience negative psychological impacts, such as body image and sexuality concerns, worry, anxiety, depression and stress.13–18 Put together, these circumstances negatively affect the patients’ self-rated health-related quality of life (HRQOL).19 Hence, the assessment and monitoring of individual patient experiences are important in breast cancer surgery because the success of aesthetic breast surgery is measured by the extent to which patients’ physical, psychological and social well-being are enhanced.7 18 20
There is strong evidence that different forms of breast surgery and reconstruction positively affect patients’ quality of life.21 Previous research has identified that evaluating patients’ outcomes of breast surgery and related psychosocial aspects through patient-reported outcome measurements (PROMs) might provide useful information for nurses, surgeons and patients.22 23 PROMs are defined as ‘any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else’24 . Previous PROM research has found that proactive PROMs, meaning that the clinicians actively review the patients’ PRO answers during therapy and use the feedback from patients to optimise the treatment and care,25 26 enable (1) earlier detection of symptoms; (2) improve communication between clinicians and patients about symptoms and HRQOL and (3) increase the person-centredness of consultation processes.27–32 A person-centred approach focuses on the care and treatment of the needs of individuals and ensures that individual preferences, needs and values guide clinical decisions and that clinicians provide care that is respectful and responsive to patients.33 Previous research in palliative care settings found that information on patients’ perception of their state through PROMs may enable clinicians to enhance person-centred care and treatment if the perspectives and experiences of patients are revealed and integrated.34
Research in PROMs has also identified several barriers to the implementation of PROMs in clinical practice.35–39 Those barriers include limited time, lack of specification of use, insufficient knowledge of clinicians on how to use PROMs, lack of capacity and electronic barriers from both patients and clinicians.35–38
The Danish government and regions have agreed to initiate a nationwide extension of PROM use in breast cancer hospitals.4 Accordingly, the Danish Breast Cancer Cooperative Group initiated a single-region PROM study on late effects in women diagnosed with breast cancer.40 This initiative is expected to become national by around 2023. However, this initiative does not include the assessment of breast surgical outcomes or systematic follow-up at the hospital, which are the core elements of this study.
PROMs in the field of breast cancer surgery have the potential to involve patients by inviting them to contribute with their preoperative and postoperative expert knowledge on their own experiences, values and concerns. PROMs may be used for systematic and person-centred follow-ups related to surgical outcomes. This has yet to be demonstrated in clinical trials.41 42
This protocol, V.1.2, 22 February 2022, describes the organisation and methodology behind a feasibility study on electronic PROMs (ePROMs) that are integrated in the treatment and care of women diagnosed with breast cancer in a plastic surgery and breast surgical outpatient setting of a tertiary university hospital. Given the emphasis on person-centred care in the organisation in which the study takes place, person-centred care is an underpinning theoretical perspective that aims to be incorporated into clinical practice; thus, the hypothesis in this multimethod study is that proactive use of ePROMs (including dialogue on satisfaction and HRQOL outcomes), promotes mutual understanding of patients’ preferences during patient trajectories at the outpatient clinic and improves patient care and communication by (1) focusing person-centred care on individual values and concerns related to surgical outcomes and psychosocial care during surgical follow-up and (2) systematic assessment of patients’ potential need for supplemental breast surgery, including reconstruction or correction, to improve patients’ well-being related to breast and body image after breast cancer. Hence, the overall aim of this study is to develop knowledge on the proactive application of ePROMs in breast surgical and breast reconstructive clinical practice.
Methodology
Study design
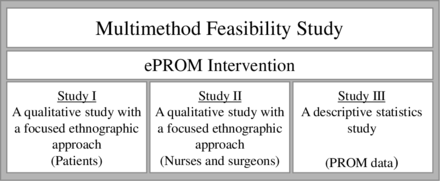
This is a multimethod, non-controlled feasibility study43 44 to investigate whether an intervention with ePROMs can be shaped to be relevant and sustainable in clinical practice. In this study, the term feasibility was inspired by Bowen et al,44 who introduce the term feasibility study for a more broad use to encompass any sort of study that can help investigators prepare for full-scale research leading to intervention study.44 We investigated and evaluated feasibility outcome variables including acceptability, demand, implementation, practicality and integration as described by Bowen and colleagues throughout three substudies (figure 1, table 1) with the following aims:
Illustration of the multimethod feasibility study, the intervention and substudies. ePROM, electronic patient-reported outcome measures.
Key areas of focus for the feasibility study inspired by Bowen et al44
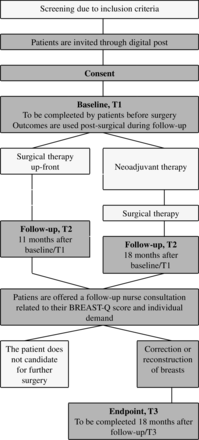
The multimethod study includes the development of an ePROM intervention with repeated collection of ePROMs at timings (T) T1, T2 and T3 using the BREAST-Q tool and proactive use of ePROMs during follow-up visits at the department, and an evaluation of feasibility (figure 2). Studies I and II are qualitative ethnographic studies exploring the user perspectives of patients, nurses and surgeons to gain insights into how the intervention can be refined. Additionally, study II is complemented with a local anonymous survey study in collaboration with department nurses and surgeons to investigate user experiences, individual activities, perceived demand, preferences and proactive application related to the ePROM intervention. Qualitative studies are guided by interpretive description (ID), an inductive methodology developed to explore clinical problems with the objective of generating insights that inform clinical practice.45 ID draws on recognised qualitative research techniques from ethnography, naturalistic inquiry, grounded theory and phenomenology but focuses on explicit research logic and flexibility, permitting researchers to apply and combine the necessary pragmatic strategies to answer the research question.45 The composition of an ID study is guided by distinctive features, including scaffolding the study, framing the study, a credible study, entering the field, constructing data, making sense of data and conceptualising findings.45 The result is a coherent, conceptual description containing understandings and illuminations of clinical phenomena, characteristics, patterns and structures in order to develop practice. The ID methodology will support understanding and knowledge related to the feasibility study outcomes.
Illustration of the ePROM-intervention flowchart. Dark boxes illustrate intervention features. T1, T2 and T3 refer to the timely specific questionnaires that are sent to the patients. ePROM, electronic patient-reported outcome measures.
Quantitative study III includes the PROM data from T1 to explore the patient population and their outcomes at baseline (figure 2).46 PROM data from T2 and T3 will be reported elsewhere. This protocol describes a feasibility study only. The evaluations of PROM data T2 and T3 will be reported elsewhere. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension47 were used to report the protocol online supplemental material 1.
Supplemental material
Study participants
Patient participants are women with newly diagnosed breast cancer, who will be included in the multimethod study from September 2021 to September 2024, and the follow-up time will end in January 2028.
Inclusion criteria:
Female patients age≥18.
Newly diagnosed breast cancer that is treated with curative surgical therapy to remove breast cancer.
The ability to speak and understand Danish to comprehend the given information, complete the study questionnaires and provide written informed consent.
Exclusion criteria:
Treated with letrozol aromatase inhibitor hormone therapy as primary treatment (nonsurgical regime, therefore outcome measures of satisfaction with surgical result are not relevant).
Not assigned digital information in the Danish Civil Registration System (figure 2).
Non-Danish speaking.
Any disability making ePROM follow-up impossible, such as blindness or mental disability, or a diagnosis of dementia.
Exclusion is assessed based on the medical record journal by a research assistant in collaboration with a breast surgeon at the department affiliated with the study. Approximately 600 women are newly diagnosed with breast cancer at the Department of Plastic and Breast Surgery of Zealand University Hospital each year. Patients will be included continuously for 3 years. A minimum sample of 900 patients is expected to be included.
For qualitative studies I and II, patient participants are purposefully sampled from consenting to the ePROM intervention using the maximum variation concept.45 Nurses and surgeons included for the qualitative studies are those whom the patients met during their visit on the day of observation by the present researcher. The patients’ visits are prebooked, and patients visit either a nurse, surgeon or a surgeon and a nurse in one consultation. An anonymous survey will be distributed to all nurses and surgeons at the outpatient clinic as part of study II.
Recruitment procedures
Patients are recruited from the Department of Plastic and Breast Surgery at a large centre of plastic and breast surgery located at a tertiary Danish university hospital. The departments’ research assistants are responsible for identifying and inviting patients who meet the inclusion criteria.
Patients eligible for inclusion are informed and invited through a digital postbox (e-Books) to the ePROM intervention.48 The invitation is supported by a 4-min video developed by the research assistant and a patient and public representative, which provides patient information about the aims of the ePROM-intervention. Furthermore, the patients receive a postcard at the outpatient clinic, which informs them about the ePROM intervention when they are diagnosed. Patients receive a link to the ePROM questionnaires in their digital postbox via the secure encrypted electronic system Research Electronic Data Capture (REDCap).49 Patients may consent or decline through invitation by mail. Patients may complete questionnaires on a PC, tablet or smartphone. The questionnaire is open for completion 14 days after invitation. After 2 days, a notification is automatically forwarded if no response is received. After 4 days, the research assistant calls and asks patients who have not responded to the invitation if they need assistance with the questionnaire. A research nurse assistant may assist with technical issues, if any. Patients included in the study can withdraw consent to participate without justification and without affecting the present or future treatment at any time. Patients withdrawing consent will be considered as ‘lost to follow-up’. Patients who decline to participate are registered within an encrypted database as non-responders. Nurses and surgeons at outpatient clinics have access to patients’ ePROM data through REDCap, including detailed responses and the total score of each questionnaire.
Strategies for the introduction of ePROMs
The introduction strategies related to this study aim to enable systematic and flexible implementation of ePROMs in an outpatient setting.15 The strategy includes establishing an ePROM-intervention support group, a nurse education programme and a surgeon education programme. As part of the strategy, ePROM intervention is described in detail within a clinical guideline developed with the ePROM-intervention support group. The guideline includes instructions for nurses, surgeons and secretaries on their specific responsibilities related to the ePROM intervention. One part of the strategy is a steering group plus education programmes for the departments’ nurses and surgeons.
Patient and public involvement
The study is supported by a patient and public representative from the Danish Cancer Society, who is an equal member of the study steering group. The representative was ‘involved throughout the design phase, for instance, contributing to the formulation of research questions and agreeing plans for dissemination of the study to participants. The representative continuously informed the study about patients’ and public priorities, experiences and preferences and the representative will participate in the analysis of data.
The ePROM steering group
The ePROM intervention is delivered by a steering group of experts who assist in the implementation of ePROMs. The group consists of an outpatient nurse from the department, a breast surgeon, a secretary, the patient and public representative, a nurse research assistant, a leading head nurse, a leading chief surgeon and a responsible nurse researcher. In addition, three external researchers are affiliated with the intervention study as supervisors and are experts in PROMs, statistics, qualitative methodology and person-centred practice.
Nurse education programme
Before the PROM intervention, all nurses at the breast surgical outpatient clinic participated in face-to-face training on the use of ePROMs. The educational sessions were guided by person-centred care theory and included a brief lecture on person-centredness and person-centred communication, which supports previous departmental education for nurses, in which person-centred values have been inherent.
Training on the application of ePROMs during consultations was mandatory and provided by departments’ clinical nurse specialist and research assistant and lasted for 4 hours. Nurses were expected to be the main users of PROM data for psychosocial support and conversations with patients, for example, on body image. Therefore, the nurses’ education was planned to be more comprehensive ‘than the surgeons’ education, and included skills training. The education programme included a broad introduction to PROMs and examples of proactive use of PROMs from other departments and research.50 51 The educational programme was planned with didactical consideration for research-based teaching and teaching for learning, and with a focus on interaction and activation during sessions with case-based learning.52 53 The training programmes included how to access the timely and relevant individual patients’ ePROMs linked to nurse consultations; how to respond to ePROMs in terms of caring for individuals with psychosocial support and symptom management; how to proactively engage in the discussion of PROM data with patients and how to document nurses’ application of PROMs in patient care. The intervention is associated with monthly 1 hour internal educational sessions that address issues related to the proactive use of ePROMs in clinical practice to improve outpatient nurses’ knowledge and skills in relevant issues such as body image-related distress.54 Nurses’ use of ePROMs is evaluated every third month using a paper questionnaire and a 1-hour dialogue with the responsible researcher.
Surgeons’ education programme
Prior to commencing the PROM intervention, surgeons from the department participated in a 1 hour mandatory education programme about the ePROM-intervention, aiming to inform about its objectives, processes rationales including how to proactively engage with ePROMS with patients. Once a month, surgeons participate in further follow-up training on PROMs, which is conducted by the responsible researcher and a clinical nurse specialist. The sessions include practical training on how to access the timely and relevant individual patients’ ePROMs linked to surgeons’ consultations as a comparison of the T1 and T2 questionnaires (table 2); how to engage with and respond to ePROMs in terms of person-centred surgical follow-up in ePROMs with patients; and how to document surgeons’ application of PROMs in the medical record journal. Didactical considerations correspond to those mentioned in the nurses’ education programmes.
Study assessment times, measures and tasks
Intervention with ePROMs
The ePROM intervention includes patients’ completion of ePROMs related to satisfaction with breasts, physical well-being, psychosocial well-being and sexual well-being, which are to be proactively applied in patients’ trajectory to monitor the individual patient’s condition and accommodate individualised psychosocial and surgical follow-up based on patient preferences and values. Over a 3-year period, the patients receive two to three questionnaires, depending on their trajectory, with treatment arms surgical therapy upfront or neoadjuvant therapy before surgical therapy (figure 2, table 1). The ePROMs are to be actively reviewed by departments’ nurses before the patient’s visit at the following times: first, prior to the patient’s 4-day postoperative control with a nurse (T1, baseline data completed before surgery); second, for the 1-year follow-up (T2, follow-up completed 11 or 18 months after surgery, dependant on treatment regime), which is initially a nurse consultation. The rationale for using baseline PROMs completed before surgery for the 4-day postoperative is: (1) The patient’s assessment of breasts before the surgery is recommended to be actively discussed with the patient in relation to the choice of breast prosthesis, bra and life with a changed body after breast cancer; (2) The baseline measurement is essential to monitor patients’ satisfaction with breasts over time, and surgical results are best evaluated at the earliest 1 year after surgery.55 Patients in the low-risk recurrence regime have standardised 1-year postoperative follow-up with nurses, where ePROMs are to be applied. Patients in high-risk recurrence regimes are not offered as standard breast surgical follow-up, but this is offered to patients through the ePROM intervention. During the second follow-up, nurses are educated to proactively use patients’ ePROMs for dialogue about patients’ perception of body image issues related to their breasts. Based on patients’ individual needs, the nurse may recommend the patient for further assessment with one of the department’s plastic surgeons, who will also have the ePROMs for comparison (table 2). Patients who accept correction or reconstruction of the breasts after their 1-year follow-up, the T2, receive a third ePROM 18 months after T2, as patients are expected to have finished their breast surgical trajectory at this point (T3, endpoint).
Data collection and measurements
The outcomes of the multimethod study relate to feasibility parameters, including acceptability, proactive use of ePROMs, demand, implementation (degree of execution), practicality and integration (perceived sustainability and fit with infrastructure), as described by Bowen et al44.44 These will be conducted through multiple measurements and outcomes in studies I to III. The data to be analysed in substudies I to III are collected as follows.
Ethnographic studies I and II
Feasibility data are collected qualitatively by exploring the user perspectives of patients, nurses and surgeons to gain insights into how the intervention can be refined. Qualitative studies I and II investigate users’ interests related to using ePROMs and practice interests that can drive or limit development. User experiences of patients, nurses and surgeons will be qualitatively explored and guided by the ID methodology for applied research.45
For studies I and II, data collection includes participant observations during patient consultations with nurses and surgeons and individual interviews with patients, nurses and surgeons to explore the application of ePROMs in clinical practice and the implications for practice. The time of the observations will follow the appointment times for the consultations (see figure 2). An observation and interview guide is developed based on the researchers’ experiences as a nurse at the department, which also allows entry into department consultations.45 The participant observations and interviews will be conducted by the first author with a focus on whether, when, how, by whom, why or why not, the ePROMs are proactively used. This work calls for critical reflection and transparency on the researcher’s positioning, degree of participation and ability to disregard the professional lens from one’s practice discipline.45 56–58 This will be reported with the results of the studies. For study II, the survey with nurses and surgeons is conducted as an online survey with questions developed specifically for this study to investigate perceptions, defined as the way in which the intervention is regarded, understood and interpreted59 as well as feasibility,46 based on the principles of applied research.45
Study III on PROMs data
PROMs are collected electronically via REDCap at time points T1, T2 and T3 (table 2). Additional baseline demographics for study III data are collected electronically via REDCap within T1 (figure 2, table 2) and include age, marital status, educational level, height, weight, body mass index and municipality.49 The PROM used for the intervention is BREAST-Q, as recommended by the International Consortium for Health Outcomes Measurement (ICHOM) standard order set for breast cancer patients to monitor PROMs following breast surgery.60 BREAST-Q was developed according to recommended guidelines through patient interviews, focus groups, an expert panel and a literature review and has undergone thorough validation with measures of high reliability which use both the paper and the electronic version.41 61–64 BREAST-Q was designed specifically for breast surgery and has preoperative and postoperative versions in modules for mastectomy, breast-conserving therapy, breast reconstruction, breast reduction and breast augmentation.65 All modules contain three subdomains, including physical, psychosocial and sexual well-being, and three subdomains on patient satisfaction, comprising satisfaction with breasts, outcome and care. No overall BREAST-Q scores are obtained. Each independent scale results in a score that is computed by adding the response items and then converting the raw sum scale score to a score from 0 to 100.66 For all BREAST-Q scales, a higher score indicates greater satisfaction or better QOL (depending on the scale). If missing data are less than 50% of the scale’s items, the mean of the completed items is inserted. Each set of questionnaires, for instance BREAST-Q questionnaire 1, takes 5–10 min to complete. Each scale is accompanied by a conversion table to calculate a total scale score of 0–100.22 66
Analysis
Qualitative studies I and II
The interviews and observations will be analysed in relation to user perspectives guided by ID. ID does not prescribe a straightforward data analysis process but relies on the pragmatic obligation of the researchers to work on data beyond initial descriptive claims towards interpretations that will enlighten the phenomenon investigated in a new and meaningful manner.67 The ID analysis aims to make sense of what has been observed and heard through an explorative process in which questions are continuously posed about the data, and answers are sought to generate explanations supported by theory.45 67 The analysis for studies I and II will be inspired by the theoretical framework of person-centred care to evaluate the feasibility of the proactive ePROM intervention by questioning whether the ePROM intervention supports the intentions on targeted, individual, psychosocial support and assessment of candidates for reconstructive and/or corrective breast surgical therapy. Specifically, the parameters of acceptability, demand, introduction, practicality and integration will be elaborated throughout the analysis (table 1).33 45 These outcomes will be informed and further analysed from the observation and interview data that is expected to add rigorous information on priorities, mechanisms and practicalities in the outpatient clinic to answer the study aims.45 67
Study III: statistical analysis
Descriptive statistics and completion rate will be calculated for all demographic variables for both responders and non-responders to the BREAST-Q questionnaire, based on data from T1 (baseline). Depending on the normality of the numerical variables, means (SD) or medians (IQR) will be calculated, while categorical variables will be expressed as proportions. Differences will be analysed using t-tests, Mann-Whitney U tests and χ2 tests. Furthermore, among responders, linear regression models will be used to identify which demographic variables are associated with the subscale scores from the BREAST-Q questionnaire. All variables will be entered into univariate and multivariate regression models to identify demographic variables that were independently associated with the questionnaire scores. Data will be analysed using the Stata software package.68 The significance level will be set at p<0.05, and all tests will be two-tailed. If applicable, sensitivity analysis using multiple imputation will be conducted on item-wise missing responses if the rate of missing data exceeds 5%.
Ethics and dissemination
The patients provide informed consent, which they can withdraw at any time. Data will be stored in REDCap and on an encrypted regional team site for sensitive personal research data. The study is designed according to the General Data Protection Regulation (GDPR) and adheres to the principles defined by the World Medical Association in the Helsinki Declaration. The use of the BREAST-Q questionnaire, authored by Drs Klassen, Pusic and Cano, was licensed by the Memorial Sloan Kettering Cancer Center, New York, USA.
The findings of this study will be submitted to international peer-reviewed journals and presented at conferences.
Ethics statements
Patient consent for publication
Acknowledgments
KH Karlse for representing patients and public into the study design. JH Prüsse for building the research database.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors STH was responsible for the initial study design and the grant application, and is responsible for supervising as project leader. STH, VJS, KML and KP did the background research and were responsible for the protocol’s in-depth development and drafting of this manuscript, including its figures and tables. LBH and BH-H helped supervise. KP, LBH and BH-H contributed with thorough revisions to the manuscript. All authors read and approved the final manuscript. STH is the guarantor.
Funding This work was supported by Region Zealand, National Cancer Plan IV (grant number 20-082, 2020). The Region Zealand has not been part of designing the study, nor will the region be part of the analysis or interpretation of data. The Department of Plastic and Breast Surgery, Zealand University Hospital and The University of Southern Denmark covers STH’s salary. KP, BHH, KML and VJS are currently not receiving a salary for this work.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.