Article Text
Abstract
Introduction Atrial fibrillation (AF) is common in patients with rheumatic mitral valve disease (RMVD) and increase the risk of stroke and death. Bi-atrial or left atrial ablation remains controversial for treatment of AF during mitral valve surgery. The study aims to compare the efficacy and safety of bi-atrial ablation with those of left atrial ablation among patients with RMVD and persistent or long-standing persistent AF.
Methods and analysis The ABLATION trial (Bi-atrial vs Left Atrial Ablation for Patients with RMVD and Non-paroxysmal AF) is a prospective, multicentre, randomised controlled study. The trial will randomly assign 320 patients with RMVD and persistent or long-standing persistent AF to bi-atrial ablation procedure or left atrial ablation procedure in a 1:1 randomisation. The primary end point is freedom from documented AF, atrial flutter or atrial tachycardia of >30 s at 12 months after surgery off antiarrhythmic drugs. Key secondary end point is the probability of freedom from permanent pacemaker implantation at 12 months after surgery. Secondary outcomes include the probability of freedom from any recurrence of atrial tachyarrhythmias with antiarrhythmic drugs, AF burden, incidence of adverse events and cardiac function documented by echocardiography at 12 months after operation.
Ethics and dissemination The central ethics committee at Fuwai Hospital approved the ABLATION trial. The results of this study will be disseminated through publications in peer-reviewed journals and conference presentations.
Trial registration number NCT05021601.
- adult cardiology
- valvular heart disease
- clinical trials
- cardiac surgery
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The trial is the first multicentre randomised controlled trial with large sample size to evaluate the efficacy of bi-atrial ablation for patients with rheumatic mitral valve disease and non-paroxysmal atrial fibrillation.
Randomisation is stratified according to centre and balanced using randomly permuted blocks (four or six patients per block), and an interactive web-based response system will be used to preserve allocation concealment.
The key secondary end point is the probability of freedom from permanent pacemaker implantation at 12 months after operation, which has been an important controversial topic.
All surgeons in this study are required to watch the video of standard Cox-Maze Ⅳ procedure and their surgical ablation procedures will be recorded before the trial, and incorrect or irregular manipulation will be reported back to surgeons, which is initiated to eliminate the impact of different tools and lesions on the results.
Introduction
Rheumatic heart disease (RHD) remains endemic among vulnerable groups in many low-income and middle-income countries, and resource-limited regions of high-income countries.1 2 About one-third of patients with RHD have atrial fibrillation (AF), with an incidence of AF almost triples every 5 years after diagnosis of RHD, and prevalence of AF is higher in severe mitral valve (MV) disease comparing with severe aortic disease.3 In patients with RHD, AF is associated with increased prevalence of heart failure, stroke, peripheral embolism and death.4–7 Especially, about 80% of the strokes in patients with RHD occur in patients with mitral stenosis and AF.8
Guidelines recommended that surgical ablation for AF could be performed without additional risk of operative mortality or major morbidity, and was recommended at the time of concomitant MV operations to restore sinus rhythm (class I, level A).9 Gillinov et al reported the addition of surgical ablation to MV surgery significantly increased the rate of freedom from AF at 1 year among patients with persistent or long-standing persistent AF in a multicentre randomised controlled trial (RCT).10 Similarly, some studies concluded that the additional surgical ablation also decreased the risk of stroke or death and increased early and long-term sinus rhythm maintenance in patients with AF and RMVD.11–13
However, there has been debate on the standard surgical ablation strategy during MV operations. Generally, bi-atrial (BA) lesion set could be created during surgical ablation because the open left atrium facilitates a BA ablation procedure, nevertheless, others believed that adding right atrial ablation had no influence on freedom from AF and conversely increased the risk of permanent pacemaker implantation. The discrepancy on the efficacy and safety between BA and left atrial (LA) ablation was also reported in the past years, whether in patients with MV disease or in patients with RMVD.14
Patients with RMVD usually have a long history and relatively severe LA remodelling, progressive pulmonary hypertension, secondary tricuspid valve regurgitation or rheumatic tricuspid valve abnormalities, which can also contribute to severe right atrial remodeling.15 16 The rationality of BA ablation is stronger in patients with RMVD and AF, however, the increased risk of permanent pacemaker implantation should not be neglected due to right atrial remodelling and fibrosis. To our knowledge, the only RCT reported a confused results that BA ablation was not superior to LA ablation in patients with RMVD and AF (p=0.09) and no conclusion on the permanent pacemaker implantation due to the limited sample.14 It might also be noted that all lesions were created by monopolar radiofrequency pen which is replaced by bipolar radiofrequency clamp in majority of lesions now.
To sum up, there is no sufficient evidence to determine the safety and potential benefits of BA ablation procedure when comparing with those of LA ablation procedure in patients with RMVD and non-paroxysmal AF. We designed this multicentre prospective RCT to compare the efficacy and safety of BA ablation with LA ablation strategies in patients with RMVD and non-paroxysmal AF.
Methods and analysis
Study objective
The ABLATION trial is designed to examine the hypothesis that for patients with RMVD and non-paroxysmal AF, BA ablation is superior to LA ablation in the probability of freedom from any recurrence of atrial tachyarrhythmias in the absence of antiarrhythmic drugs, and non-inferior to LA ablation in the probability of freedom from permanent pacemaker implantation.
Study design
ABLATION is a multicentre, open-label, two-arm, single-blind, parallel RCT designed to compare the efficacy and safety of BA ablation with those of LA ablation among patients with RMVD and non-paroxysmal AF.
The study plans to recruit patients from 19 large academic cardiac centres all over Chinese mainland. Patients aged ≥18 years, with RMVD and non-paroxysmal AF who underwent MV surgery concomitant surgical ablation will be eligible for enrolment. RMVD is determined by history of acute rheumatic fever, valve morphology, echocardiographic findings and pathological diagnosis. Echocardiographic and intraoperative findings of leaflet thickening and retraction, commissural fusion and/or chordal fusion and shortening are considered as RMVD.17
Exclusion criteria include paroxysmal AF, degenerative or ischaemic MV disease, previous catheter ablation or surgical ablation for AF, surgical management of hypertrophic obstructive cardiomyopathy, absolute contraindications for anticoagulation therapy, LA thrombosis, chronic obstructive pulmonary disease, uncontrolled hypothyroidism or hyperthyroidism, LA diameter >70 mm, right ventricular dysfunction or moderate-to-severe tricuspid regurgitation or pulmonary artery systolic pressure >60 mm Hg, coronary artery bypass grafting required for participants with coronary heart disease (see box 1 for details).
The inclusion and exclusion criteria for the study
Inclusion criteria
Age ≥18 years.
Persistent or long-standing persistent AF documented by medical history or direct electrocardiograpy.
Concomitant cardiac surgery involves at least mitral valve surgery.
Agree to perform ablation procedure.
Exclusion criteria
Paroxysmal AF.
Degenerative or ischaemic mitral valve disease.
Evidence of active infection.
Previous percutaneous catheter ablation or surgical ablation for AF.
Surgical management of hypertrophic obstructive cardiomyopathy.
Absolute contraindications for anticoagulation therapy.
Left atrial thrombosis (not including left atrial appendage thrombosis alone).
Chronic obstructive pulmonary disease (forced expiratory volume in 1 s <30% anticipated value).
Uncontrolled hypothyroidism or hyperthyroidism.
Mental impairment or other conditions that may not allow participants to understand the nature, significance and scope of study.
Left atrial diameter >70 mm.
Right ventricular dysfunction (TAPSE <16) or moderate-to-severe tricuspid regurgitation or pulmonary artery systolic pressure (estimated by echocardiography) >60 mm Hg.
Coronary artery bypass grafting is required for participants with coronary heart disease.
Previous cardiac surgery.
Refuse to participate in this study.
AF, atrial fibrillation; TAPSE, tricuspid annular plane systolic excursion.
Information about trial objective, design, interventions and potential risks and benefits will be introduced thoroughly to all potential participants. They are encouraged to ask questions to study personnel and discuss the trial with family or friends prior to decision to participate. A written consent is mandatory prior to randomisation. The study is approved by ethics committees in Fuwai Hospital and has been registered at ClinicalTrials.gov, identifier NCT05021601. All participating sites accepted the central ethics approval or obtained approval by the local ethics committee. The ABLATION trial began recruitment in May 2022 and is expected to complete recruitment by the end of April 2024 and follow-up will be completed by the end of April 2025 (figure 1).
A Gantt plot showing the progress of this study.
Randomisation
Eligible patients were randomised (1:1) to BA ablation group or LA ablation group. An interactive web-based response system will be used to preserve allocation concealment. Randomisation is stratified according to centre and balanced using randomly permuted blocks (four or six patients per block). Surgeons are aware of randomisation results, however, participants and research staff are all blinded to the randomisation schemes.
Treatment arms
The operation will be performed under cardiopulmonary bypass under general anaesthesia, and preoperative transoesophageal echocardiography will be used to exclude intracardiac thrombi. Except for MV operations, participants randomly assigned to BA ablation group will receive BA ablation, and who randomly assigned to LA ablation group will receive LA ablation. Unified ablation tools and lesion sets are applied during surgical ablation, and the principles of using ablation tools are strictly followed.
BA group
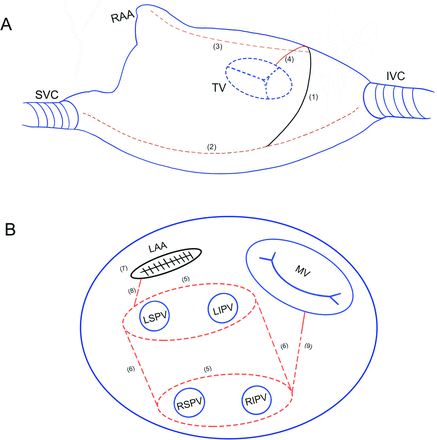
In this arm, Cox-Maze IV lesion sets are created. The detailed lesions were reported by Ruaengsri et al and Cox et al.18 19 After the initiation of cardiopulmonary bypass, a vertical right atriotomy is made extending from the intra-atrial septum up towards the atrioventricular groove near the free margin of the heart. And then, from the inferior aspect of the incision, the radiofrequency bipolar clamp is used to create ablation lines up to the superior vena cava and down towards the inferior vena cava. Then the right atrial appendage is clamped by bipolar clamp from the side of the right atrial vertical incision near the atrioventricular groove towards the tip of the right atrial appendage.19 The transpolar or irrigated radiofrequency pen is used to create an endocardial ablation line from the superior aspect of this vertical right incision down onto the tricuspid annulus at the 2 o’clock position (figure 2A). In order to ensure transmurality, overlap epicardial ablation can be created by radiofrequency pen in line with endocardial ablation line when right atrium wall is thickened significantly.
Schematic surgical lesion sets. (A) Right atrial lesions. (B) Left atrial lesions. The solid black lines indicate the surgical incision, and the dotted red lines indicate the ablation lines by radiofrequency bipolar clamp, and the solid red lines indicate the ablation lines by radiofrequency pen. (1) A vertical right atriotomy extending from the intra-atrial septum up towards the atrioventricular groove; (2) line from SVC to IVC; (3) the RAA is clamped by bipolar clamp from the side of the right atrial vertical incision near the atrioventricular groove towards the tip of the RAA; (4) an endocardial ablation line from the superior aspect of the vertical right incision down onto the tricuspid annulus at the 2 o’clock position; (5) PVI; (6) isolation of the posterior left atrium; (7) management of the LAA; (8) left superior PV to the LAA; (9) mitral isthmus line. IVC, inferior vena cava; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; PVI, pulmonary veins isolation; RAA, right atrial appendage; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; TV, tricuspid valve.
At left atrium, right pulmonary veins can be isolated by radiofrequency bipolar clamp first, and other LA lesions are performed on the arrested heart after aortic cross-clamping. After the ligament of Marshall division, left pulmonary veins (PVs) are isolated by radiofrequency bipolar clamp. After left atrial appendage (LAA) is amputated, LA roof and floor ablation lines are created to connect with bilateral pulmonary vein isolation (PVI) loops by radiofrequency bipolar clamp. In addition, ablation lines are created to connect right PVI loop towards to the posterior mitral annulus, as well as left superior PV to the LAA by radiofrequency bipolar clamp. Finally, a radiofrequency pen is used to complete the endocardial mitral isthmus lesion, and to perform an epicardial radiofrequency ablation across the coronary sinus in line with the endocardial mitral isthmus lesion created by radiofrequency pen (figure 2B).
LA group
As mentioned above, in this arm, participants are performed LA ablation alone on the arrested heart after aortic cross-clamping (figure 2B).
Each site is effectively ablated at least 3 times with radiofrequency clamp without releasing the radiofrequency clamp. When using dry radiofrequency clamp, the ablation peak value of conductance curve no less than 15 and the time of each ablation to the transmural impedance value no longer than 10 s is determined as effective ablation. The first time to reach the transmural impedance value must be no less than 3 s using irrigated radiofrequency bipolar clamp. Endocardial ablation by radiofrequency pen is performed twice at each 1 cm long distance for no less than 15 s. MV surgery and other surgery (such as aortic valve surgery) are performed after ablation. All surgeons in this study are required to watch the video of standard Cox-Maze Ⅳ procedure and their surgical ablation procedures will be recorded before the trial, and incorrect or irregular manipulation will be reported back to surgeons, which is initiated to eliminate the impact of different tools and lesions on the results.
Study end points
The primary end point is the probability of freedom from any recurrence of atrial tachyarrhythmias off antiarrhythmic drugs at 12 months after operation documented by 3-day Holter monitoring. Atrial tachyarrhythmia recurrence will be considered when any episode of AF, atrial flutter or atrial tachycardia is sustained ≥30 s on ECG monitoring after the blanking period.20 The first 3 months after operation is considered as blanking period.
The key secondary end point is the probability of freedom from permanent pacemaker implantation at 12 months after operation, that is, the percentage of participants who do not have a new implanted permanent pacemaker.
The secondary end points are the probability of freedom from any recurrence of atrial tachyarrhythmias with antiarrhythmic drugs, AF burden, incidence of adverse events (including cardiac death, stroke, hospitalisation for heart failure, hospitalisation for embolism events or major bleeding events) and cardiac function documented by echocardiography at 12 months after operation. All end points are listed in box 2.
End points in this trial
Primary end point
The probability of freedom from any recurrence of atrial tachyarrhythmias without AADs at 12 months after operation.
Key secondary end point
The probability of freedom from permanent pacemaker implantation at 12 months after operation.
Secondary end points
The probability of freedom from any recurrence of atrial tachyarrhythmias with AADs at 12 months after operation.
Burden of AF (evaluating with 3-day Holter monitoring at 12 months after operation).
Incidence of adverse events (including cardiac death, stroke, hospitalisation for heart failure, hospitalisation for embolism events or bleeding events).
Cardiac function documented by echocardiography at 12 months after operation.
AF, atrial fibrillation; AADs, antiarrhythmic drugs.
Hospital and surgeon selection
This is a multicentre study, and there are strict requirements for collaborative hospitals and surgeons. The annual volume of surgical ablation concomitant MV operations of hospital should be >100 cases; surgeons should be proficient in the standard use of radiofrequency bipolar clamps and pens, and the total volume of surgical ablation should be >20 cases.
Postablation management
After the operation, anticoagulation with warfarin is routinely initiated in all participants in the early postoperative period for 3 months, and participants with cardiac mechanical prosthetic valve need lifetime anticoagulation therapy. However, antiarrhythmic drugs are prescribed for 2 months only if AF or atrial flutter occurs during perioperative period.
Data collection and follow-up
A web-based data entry system has been established on the Chinese Cardiac Surgery Registry (CCSR) website (http://ccsr.cvs-china.com).21 This web-based CCSR data collection platform uses a high-level secure socket layer. The ABLATION trial uses this paperless data submission system for data collection, follow-up and management. All enrolled hospitals participating in the study are authorised to access the data submission system. The dataset for this study includes the following four modules: subject screening, informed consent and randomisation, baseline in-hospital information, 3-month, 6-month and 12-month follow-up data.
For baseline data, participating sites may directly import the in-hospital data into the CCSR database, including patient characteristics, comorbidities, oral medications, preoperative examination (24-hour Holter monitoring, echocardiography, thyroid function, etc), surgical information, postoperative complications and discharge data. Baseline data should be completed within 14 days after discharge.
All the follow-ups are completed by a professional team blinded to the group allocation. The 3-month and 6-month follow-ups are completed via a remote video interview using social media. All video interviews are recorded. Twenty-four-hour Holter monitoring information and questionnaire are collected at 3-month follow-up, and 3-day Holter monitoring information and questionnaire are collected at 6-month follow-up. For the 12-month follow-up, a face-to-face visit is conducted in hospital or via a remote video interview. The study participants will be contacted in advance to confirm the type of 12-month follow-up. Three-day Holter monitoring information, questionnaire and echocardiography are collected at 12-month follow-up. The questionnaire includes questions on subject survival status, cardiac function classification, stroke, peripheral thromboembolic events, hospitalisation for heart failure, bleeding events, medication use and permanent pacemaker implantation. The 3-day Holter monitoring devices are mailed to participants for wearing at 6-month and 12-month follow-up. After wearing, they are sent back to the project team for data analysis. If permanent pacemaker is implanted in a participant during follow-up, we will record the date and reason that the participant’s pacemaker is implanted by questionnaire. During participant enrolment, we inform participants that if they have a subsequent readmission for treatment, they need to save and submit their case information to us during follow-up. In addition, we will record and analyse the time taken by the pacing rhythm by 3-day Holter monitoring at 6-month and 12-month follow-up. All the follow-up information will be uploaded to the web-based CCSR data collection platform. In addition, we request participants to have ECG tests at each follow-up and at any time after surgery if they have cardiac symptoms. The 3-day Holter monitoring, 12-lead ECGs and echocardiograms will be analysed by a core lab blinded to the group allocation.
Statistical analysis plan
Sample size calculation
The calculation of the sample size is based on the primary end point and the key secondary end point according to previously published data and our own clinical experience. The primary end point of the study is the probability of freedom from any recurrence of atrial tachyarrhythmias at 12 months after operation. It is estimated that the probability of freedom from atrial tachyarrhythmias at 12 months in the LA group is 70%10 17 22 and that in the BA group is 85%.10 17 23 Therefore, a sample size of 131 patients (per group) is needed to provide 90% power based on a one-sided Z test with pooled variance and a significance level of 0.05 (one-sided).
The key secondary end point of this study is the probability of freedom from permanent pacemaker implantation at 12 months. It is estimated that the probability of freedom from permanent pacemaker implantation at 12 months in the LA group is 97%.14 24 Considering the feasibility of clinical studies, the non-inferiority margin is determined as −5%.25–27 Therefore, a sample size of 144 patients (per group) is needed to provide 80% power based on a one-sided Z test with pooled variance and a significance level of 0.05 (one-sided).
As mentioned above, both primary and key secondary end points should be considered. Therefore, 144 patients per group are required. When considering a withdrawal rate of 10%, 320 patients are required to be randomly assigned into two groups in a 1:1 allocation.
Statistical analysis
A hierarchical testing procedure is applied to the primary and key secondary end points to preserve the overall type I error of 5%. The key secondary end point would only be tested (at significance level 5%) if the test for the primary end point is statistically significant (significance level 5%). Non-inferiority will be concluded if the lower limit of the 95% CI for the difference in proportion of participants achieving freedom from atrial tachyarrhythmias is greater than the –5% non-inferiority margin.
We will use frequencies with percentages to describe categorical variables, and means with SD or medians with IQRs to describe continuous variables. We will compare baseline participant characteristics and end points between the LA and BA groups using χ2 tests for categorical variables and Student’s t-tests for continuous variables. The Kaplan-Meier estimator will be applied to evaluate the probability of freedom from any recurrence of atrial tachyarrhythmias, and the log-rank test will be used for the evaluation of between-group variance. The primary and key secondary end points are determined on the basis of the intention-to-treat principle. In addition, a per-protocol analysis is also performed, which includes participants who complete their assigned treatments as scheduled. All statistical tests are one-tailed with a significance level of 0.05.
Patient and public involvement
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics and dissemination
Ethics and governance approvals were obtained by the central ethics committee at Fuwai Hospital. Written informed consent will be obtained from all study participants prior any study-specific assessments. The results of this study will be disseminated through publications in peer-reviewed journals and conference presentations.
Discussion
There has been a long-time debate about BA ablation or LA ablation alone for concomitant surgical ablation during MV surgery and relevant guidelines have not given explicit recommendations about it.9 20 28 After a period of relative neglect, there has been a resurging interest in RHD worldwide over the past decade.2 Comparing degenerative MV disease, RMVD often has a chronic condition with immune and inflammatory cells attack, which tends to affect the right atrium apart from left atrium, including pulmonary hypertension or tricuspid regurgitation.29 Previous studies showed that structural and electrical remodelling uniformly distributed across both atria in RMVD.15 16 Which lesion set should be preferred to be created during surgical ablation in patients with RMVD and AF? The current literature provides insufficient evidence to address this important clinical issue. Few studies with limited sample size have reported different results of surgical ablation with diverse lesion sets in patients with RMVD and non-paroxysmal AF.11 13 14 30–33 Therefore, to the best of our knowledge, ABLATION trial is the first multicentre RCT with large sample size to evaluate the efficacy of BA ablation for patients with RMVD and non-paroxysmal AF.
Whether the additional right atrial ablation to LA ablation increases the risk of permanent pacemaker implantation has been an important controversial topic. Right atrial structural remodelling including atrial fibrosis may influence sinoatrial node function or contribute to sinoatrial block.34 This condition might be even worse with right atrial lesions are created. However, Cox et al believe that there are many reasons for permanent pacemaker implantation after surgery, but standardised right atrial ablation set do not increase the risk of permanent pacemaker implantation.35 36 Other studies displayed LA fibrosis or dilation was associated with sinus node dysfunction requiring pacemaker implant.37 38 Nevertheless, previous meta-analyses showed that the additional right atrial ablation increased the risk of permanent pacemaker implantation.24 In order to clarify this topic, we also evaluate the incidence of permanent pacemaker implantation in ABLATION trial. We regard the probability of freedom from permanent pacemaker implantation at 12 months after operation as the key secondary end point. A hierarchical testing procedure is applied to the primary and key secondary end points to preserve the overall type I error of 5%, which were widely used by previous studies.39 40 If the hypothesis with end point on permanent pacemaker implantation is supported by the result of the ABLATION trial, it is believed that this conclusion can be applied in other MV diseases which have less right atrial remodelling.
This is an investigator-initiated study, and false positive can be controlled less strictly because the issue of false negative is equally important. From the overall study design, a hierarchical testing procedure is applied to the primary and key secondary end points, the hypothesis test of the key secondary end point can be carried out only if the primary end point reached positive. Therefore, it is very important to obtain the positive result of the primary end point with greater power, and then to carry out the sequential test of the key secondary end point. Once the hypothesis test of primary end point fails, there is no need for hypothesis test of key secondary end point. In order to take into account this goal, we tend to choose a greater power (90%). Therefore, we chose a significance level of one-sided 0.05 and 90% power.
In order to reduce the missed diagnosis rate of recurrent paroxysmal AF, we assess the primary end point of the probability of freedom from any recurrence of atrial tachyarrhythmias by means of 3-day continuous Holter monitoring at 6-month and 12-month follow-up after surgery, which was used by previous study.10 In addition, 24-hour Holter monitoring will be performed at 3-month follow-up, and 12-lead ECGs will be performed at each follow-up, and for participants who have AF episode or other suspicious cardiac symptoms, all ECGs will be analysed at any time point after surgery.
It is common that every surgeon has the surgical option based on their understanding on AF.41According to the guideline,42 all participated surgeons in ABLATION trial are experienced and undergo the training and education to improve their understanding of AF, complete lesion set and every reliable lesion. All surgeons are required to watch the video of standard Cox-Maze Ⅳ procedure and their surgical ablation procedures will be recorded before the trial is initiated. Incorrect or irregular manipulation will be reported back to surgeons. Compared with previous study,43 unified ablation tools and matched lesion set in every group will be emphasised and implemented in order to eliminate the impact of different tools and lesions on the results. In addition, it is possible that the severe right atrial remodelling exists when right ventricular dysfunction or moderate-to-severe tricuspid regurgitation or severe pulmonary hypertension, which may contribute to the substrate of AF. In these patients, LA ablation alone is unethical, thus, these patients are not enrolled in ABLATION trial.
In conclusion, the ABLATION trial is designed to examine the efficacy and safety of BA ablation procedure versus LA ablation procedure with unified ablation tools and matched lesion set in patients with RMVD and non-paroxysmal AF. The findings from this trial may help determine an optimal ablation lesion set to further improve the prognosis of patients with RMVD and non-paroxysmal AF.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
References
Footnotes
CY and HL contributed equally.
Contributors CY, HL, YW, SC, YZ, ZZ: study concept and design; CY, HL, ZZ: drafting the initial manuscript and critical revision of the paper. All authors read and approved the final manuscript.
Funding This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-063).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.