Article Text
Abstract
Objectives Previous studies were unable to estimate the dynamics of smoking status in the US elderly general population, and no study has assessed the benefit of quitting in terms of resultant gains in life expectancy. We proposed a novel method to estimate the per cent of quitting in remaining lifetime, successful quitting and relapse, as well as life expectancy by participants’ baseline smoking status.
Design Longitudinal cohort.
Setting US community-dwelling population.
Participants Respondents from the Medicare Health Outcome Survey Cohort 15 (baseline 2012, follow-up 2014). We included respondents who were aged ≥65 years and alive at the baseline and participated in the baseline survey (n=164 597).
Primary and secondary outcome measures
Attempt quitting, successful quitting, relapse rates and life expectancy by smoking status at age 65–95 years.
Results Among daily smokers aged 65 years, 61% would attempt to quit during their remaining lifetime, and 31% would quit successfully. Among some days smokers aged 65 years, 69% would attempt to quit during their remaining lifetime, and 37% would quit successfully. Among recent ex-smokers aged 65 years, 53% would relapse. Life expectancy at age 65 years was 20.0 (SE=0.27), 17.2 (SE=0.30), 16.2 (SE=0.29) and 15.9 (SE=0.29) years for long time non-smokers, recent ex-smokers, some days smokers and daily smokers, respectively. Although recent ex-smokers had a higher 2-year mortality than current smokers, those who quit up to 77 years (77 years for men and 87 years for women) had a significantly longer (p<0.05) life expectancy. Sensitivity analysis demonstrated that the model assumptions had a relatively small impact on estimates with a maximum relative bias within ±7%.
Conclusions This study provides detailed information regarding the dynamics of smoking status in an understudied and growing population and demonstrates the benefit of smoking cessation on life expectancy. Future research should focus on understanding specific predictors of smoking cessation.
- statistics & research methods
- geriatric medicine
- public health
Data availability statement
Data may be obtained from a third party and are not publicly available. This study is a secondary data analysis using the Limited Data Set (LDS) of the HOS from the US Centers for Medicare & Medicaid Services (CMS). The dataset contains potentially identifying or sensitive patient information (eg, participants’ zip code, date of birth, date of death, etc). A signed Data Use Agreement (DUA) with CMS is required to obtain LDS data files (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/HOS). In order to request a LDS file, investigators must follow the instructions on this link: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/EPPEpilot-LDSS.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The large sample size of this study enables us to obtain estimates, with good reliability, at each of the 2-year age intervals from age 65 years to 95 years.
Our method relied on the assumption that all relapses occurred within 2 years of abstinence and older longtime non-smokers do not initiate smoking or experience relapse.
We conducted extensive sensitivity analyses that demonstrated that the impact of the main model assumption on all estimates was small.
This analysis did not examine factors (other than age and gender) that affected quitting and relapse or the reasons for quitting and relapse.
Introduction
In the USA, smoking is the leading cause of preventable death.1 Yet, despite substantial evidence that older persons benefit from quitting smoking in terms of both morbidity and mortality, research on smoking cessation tends to focus on younger adults.2 3 Older adults differ from younger adults with regard to smoking prevalence, quit attempts and relapse.3 4 Findings from nationwide surveys of the US general population indicate that the smoking prevalence is lower among older adults than among younger adults.3 5–7 As an example, based on the 2016 National Health Interview Survey (NHIS), 8.8% of persons aged 65 years and older were current smokers compared with 17.1% of persons aged 18–64 years.7 However, compared with younger smokers, older smokers are less likely to attempt quitting in the preceding 12 months.6 Regarding the impact of smoking on life expectancy, using data from the 2009 Behavioural Risk Factor Surveillance System (BRFSS) and the NHIS, life expectancy at age 18 years was estimated to be 62.5 years for non-smokers (never or former smokers) and 53.6 years for current smokers, resulting in an average loss of 9.0 years to smoking for smokers aged 18 years.8
Previous investigations used data from nationwide, population-based, cross-sectional surveys that relied on retrospective assessments to ascertain dynamics of smoking, such as attempts to quit, successful quitting and smoking relapse.5–7 9 10 These assessments were based on the following four questions from the NHIS, the National Health and Nutrition Examination Survey (NHANES) and the BRFSS: (1) “Have you smoked at least 100 cigarettes in your entire life?” (2) “Do you now smoke cigarettes every day, some days, or not at all?” (3) “During the past 12 months, have you stopped smoking for longer than a day because you were trying to quit smoking?” and (4) “How long has it been since you quit smoking cigarettes?” For example, respondents who answered ‘yes’ to question (3) were classified as having a recent quit attempt.6 7 Respondents who answered ‘every day’ or ‘some days’ to question (2) and ‘yes’ to question (3) were classified as having a smoking relapse.5 Successful quitting was measured by calculating the quit ratio which is the ratio of former smokers, those who answer ‘yes’ to question (1) and ‘not at all’ to question (2), to ever smokers, those who answer ‘yes’ to question (1).6 7 9 However, this approach cannot provide estimates of the per cent of quitting and successful quitting for current smokers and per cent of relapse for ex-smokers because the appropriate denominators for the calculation of percentages cannot be determined. In addition, estimates based on these questions are subject to recall bias as well as selective survival bias.
It is also difficult to quantify the impact of smoking on mortality and the benefits of quitting in terms of years of life lost due to smoking and gains in life years after quitting.11 To our knowledge, no study has estimated gains in life expectancy after quitting in the older US general population. A number of different reasons exist as to why this is not a straightforward analysis. For example, health risks, in the form of chronic diseases and premature death, may become clinically manifest only after many years of smoking. Similarly, the impact of quitting, in the form of a decrease in mortality, may not be apparent until many years after having quit. Conversely, being in poor health or having chronic conditions usually is the main reason to quit smoking,12 13 and, therefore, recent ex-smokers might have a higher mortality several years after quitting as compared with current smokers.11 Additionally, smoking status is not a permanent state and may change throughout the lifespan. A person may attempt to quit and relapse many times.14 15 Because of these potential scenarios, estimating life expectancy for individuals based on their baseline smoking status should account for transitions between different smoking statuses during their remaining lifetime.16 However, previous analyses of losses in life expectancy due to smoking assumed that smoking status would be unchanged throughout the remainder of expected lifetime for both smokers and non-smokers, and, therefore, this assumption would likely overestimate years of life losses to smoking.8 17 18
Ideally, these estimates should be from a large prospective cohort that is representative of the general population and records participants’ smoking status on a regular basis over many decades until death. Due to the high data requirements, we developed a novel method that used a single current smoking status question, question (2) above, from a large, cohort sample of the US elderly population with a relatively short follow-up interval. The present study describes and applies this method to estimate dynamics of smoking status of US older adults aged 65 years or older using a large, national representative legacy dataset. There are two specific aims: (1) to estimate the percentages of quitting during the remaining lifetime and successful quitting among current smokers and the percentages of relapse among recent ex-smokers (defined as <2 years of abstinence) and (2) to estimate life expectancy at age 65–95 years by respondents’ baseline smoking status. We examined whether life expectancy increased after quitting. Finally, to assess the validity of our estimates, we conducted a sensitivity analysis by examining the impact of the model assumptions on our estimates.
Methods
In this study, the term ‘quitting’ refers to a participant who reported smoking previously and does not currently smoke. ‘Successful quitting’ refers to an ex-smoker who has been abstinent from smoking for at least 2 years and remains a non-smoker until death. ‘Relapse’ refers to a person who reported not smoking previously and is smoking now.
Data and measures
The data were obtained from the Medicare Health Outcome Survey (HOS), a nationwide survey of Medicare beneficiaries.19 Each year, the HOS randomly selects a cohort of Medicare beneficiaries who voluntarily enrolled in Medicare Advantage private health plans. The selected individuals who completed a baseline survey are resurveyed 2 years later. We used the Medicare HOS cohort 15 whose baseline data were collected in 2012 and follow-up data were collected in 2014. This dataset contains date of death if death occurred by 31 January 2015. We included respondents who were aged 65 years or older and alive at the baseline and participated in the baseline survey. The total sample was 164 597 (online supplemental table S1). Among them, 100 290 (61%) were alive at follow-up and completed the follow-up survey, and 64 597 (39%) did not participate in the follow-up survey, including 26 111 (16%) who died and 38 196 (23%) who were alive, but did not complete the survey. An additional 88 participants died after completing the follow-up survey. The average time from the baseline to follow-up survey was 730.3 days (IQR 700–730 days). The average follow-up time (from baseline to death or to 31 January 2015) was 901.1 days (IRQ 932–1099 days).
Supplemental material
The Medicare HOS includes only one question on current smoking status. The survey did not ask respondents about their lifetime smoking status nor recent quit attempt. At both baseline and follow-up surveys, the HOS askes respondents “Do you now smoke every day, some days, or not at all?”20 We used this question to classify respondents as daily smokers, occasional smokers and non-smokers. Of the 164 597 individuals who participated in the baseline survey, 158 964 (96.6%) answered the smoking question; of the 100 290 individuals who participated in the follow-up survey, 93 905 (93.6%) answered the smoking question (online supplemental table S1).
Statistical analysis
We proposed a smoking transition model that classified non-smokers into recent ex-smokers and longtime non-smokers based on their answers to the current smoking question at the baseline and 2 years later. The microsimulation method was used to project individuals’ future smoking status until death through a sequence of independent trials in a first-order Markov process based on multistate models and to estimate their expected number of remaining life years (ie, life expectancy).21 22
Multistate models were used to estimate probabilities of transferring between different smoking states.16 22 Because the baseline and follow-up surveys were 2 years apart, we constructed a multistate model in 2-year age intervals, at ages 65, 67, … years. To illustrate the method, we describe a Markov process with k transient states ( ) for k levels of smoking status and one absorbing state (
) for k levels of smoking status and one absorbing state ( ) for dead. Let
) for dead. Let  be the transition probability from state
be the transition probability from state  at age t to state
at age t to state  at age
at age  . These transition probabilities satisfy linear dependence:
. These transition probabilities satisfy linear dependence:  for all i. Because
for all i. Because  is the absorbing state,
is the absorbing state,  for
for  and
and  .
.
Using the current smoking status question, we applied a multistate model with three transient states,  for ‘smoking daily’, ‘smoking occasionally’ and ‘non-smoking’, and one absorbing state (
for ‘smoking daily’, ‘smoking occasionally’ and ‘non-smoking’, and one absorbing state ( ) for dead (figure 1, model A). This model has a transition matrix
) for dead (figure 1, model A). This model has a transition matrix
Transition models of smoking status.

For example,  and
and  are probabilities of quitting for daily smokers, and some days smokers, respectively;
are probabilities of quitting for daily smokers, and some days smokers, respectively;  and
and  are probabilities of relapse for daily smokers and some days smokers, respectively and
are probabilities of relapse for daily smokers and some days smokers, respectively and  and
and  are probabilities of dying in 2 years for daily smokers, some days smokers and non-smokers, respectively.
are probabilities of dying in 2 years for daily smokers, some days smokers and non-smokers, respectively.
In model A, the ‘non-smoking’ state includes recent ex-smokers, longtime ex-smokers and never smokers. This model assumes the same relapse and mortality rates for them. Although recent ex-smokers compose a very small proportion of the ‘non-smoking’ group, nearly all relapses are from those with <2 years of abstinence.17 23 24 Also, recent ex-smokers might have a higher mortality rate than longtime ex-smokers and never smokers. Therefore, model A may be invalid. The main problem is that the Medicare HOS did not ask respondents about their lifetime smoking status. Therefore, we were unable to separate non-smokers into never smokers and former smokers.
To solve this problem, we partitioned the ‘non-smoking’ state,  , into two mutually exclusive states:
, into two mutually exclusive states:  for ‘recent non-smoking’ and
for ‘recent non-smoking’ and  for ‘longtime non-smoking’ (figure 1, model B). Because never smokers and longtime ex-smokers would be unlikely to start smoking at an advanced age of 65 years or older and nearly all relapses (about 99%) were within 2 years of abstinence,23–26 we assumed all relapses occurred within 2 years of abstinence and older longtime non-smokers do not initiate smoking or experience relapse, that is,
for ‘longtime non-smoking’ (figure 1, model B). Because never smokers and longtime ex-smokers would be unlikely to start smoking at an advanced age of 65 years or older and nearly all relapses (about 99%) were within 2 years of abstinence,23–26 we assumed all relapses occurred within 2 years of abstinence and older longtime non-smokers do not initiate smoking or experience relapse, that is,  . Based on this assumption, model B has a transition probability matrix
. Based on this assumption, model B has a transition probability matrix

For model B, the recent non-smoking measure was operationalised as smoking at baseline and not smoking at follow-up. The longtime non-smoking measure was operationalised as not smoking at both baseline and follow-up.
All transition probabilities ( and
and  ) in model A at a given age between states
) in model A at a given age between states  and
and  can be estimated from the HOS data (details are in online supplemental appendix A1).16 For model B, six transition probabilities (
can be estimated from the HOS data (details are in online supplemental appendix A1).16 For model B, six transition probabilities (
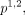


 and
and  ) are available from model A directly. The remaining eight transition probabilities,
) are available from model A directly. The remaining eight transition probabilities, 





 and
and  , can be estimated based on assumptions of model B (details are in online supplemental appendix A2).
, can be estimated based on assumptions of model B (details are in online supplemental appendix A2).
Microsimulations
We projected the future smoking status of each individual in a synthetic cohort of persons using the microsimulation method.21 22 For an age cohort of 1 000 000 individuals of a given initial state  at starting age x, using the estimated transition probabilities of model B, we simulated each individual’s smoking state at age
at starting age x, using the estimated transition probabilities of model B, we simulated each individual’s smoking state at age 
 iteratively until all individuals died. Per cent of quitting in the remaining lifetime was estimated as the proportion ever entering the recent non-smoking state (
iteratively until all individuals died. Per cent of quitting in the remaining lifetime was estimated as the proportion ever entering the recent non-smoking state ( ) for a cohort of current smokers (
) for a cohort of current smokers ( or
or  ) at the starting age x. Per cent of successful quitting was estimated as the proportion entering the longtime non-smoking state (
) at the starting age x. Per cent of successful quitting was estimated as the proportion entering the longtime non-smoking state ( ) for a cohort of current smokers. Per cent of relapse was estimated as the proportion entering the current smoking states (
) for a cohort of current smokers. Per cent of relapse was estimated as the proportion entering the current smoking states ( or
or  ) for a cohort of recent ex-smokers (
) for a cohort of recent ex-smokers ( ).
).
Life expectancy is estimated as the average number of years from the starting age to age of death for a cohort of individuals in a given initial state  at starting age x. If death occurred during the age interval from
at starting age x. If death occurred during the age interval from  to
to  , average years to death is
, average years to death is  , where a is the proportion of time lived in the 2-year age interval for persons who died during the interval. Assuming a constant mortality rate during an age interval, it can be shown that
, where a is the proportion of time lived in the 2-year age interval for persons who died during the interval. Assuming a constant mortality rate during an age interval, it can be shown that  , where
, where  is probability of death during the age interval. When P is small and close to 0,
is probability of death during the age interval. When P is small and close to 0,  , otherwise,
, otherwise,  .
.
The SEs of all estimates were derived from microsimulation and includes the random variation of individuals’ outcomes conditional to transition probabilities and imprecision of the estimated transition probabilities (ie, first-order and second-order Monte Carlo uncertainty).22 27
Sensitivity analysis
Our estimates relied on the assumption that all relapses occurred within 2 years of abstinence and no relapse for longtime non-smokers. This assumption may lead to underestimation of successful quitting. To assess the impact of this assumption, we conducted a sensitivity analysis by examining a model that allows relapse for non-smokers who had not smoked in the past 2+ years (figure 1, model C). Because relapse rates decreased with years of abstinence,5 25 26 28 we assumed that all relapses for those who had not smoked in the past 2+ years occurred between years 2 and 4 of abstinence and there was no relapse after 4+ years of abstinence. Extending from model B, and let states 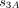 ,
,  and
and  be non-smoking for <2 years, 2–4 years and ≥4 years, respectively, the model C has a transition matrix
be non-smoking for <2 years, 2–4 years and ≥4 years, respectively, the model C has a transition matrix

To estimate transition probabilities of model C, we made following two assumptions:
(1) We assumed the probabilities of transferring from state  to states
to states  and
and  are in the form of
are in the form of  and
and  , respectively, where RR is the risk ratio of relapse for state
, respectively, where RR is the risk ratio of relapse for state  relative to state
relative to state  . Data from previous studies showed that the relapse rate for those with 2+ years of abstinence was between 5% and 13% of that for those with ≤2 years of abstinence.23–26 28 We used RR=0.05 (scenario A) and 0.15 (scenario B) as lower bound and upper bound, respectively.
. Data from previous studies showed that the relapse rate for those with 2+ years of abstinence was between 5% and 13% of that for those with ≤2 years of abstinence.23–26 28 We used RR=0.05 (scenario A) and 0.15 (scenario B) as lower bound and upper bound, respectively.
(2) We expected that the mortality rates for those who had quit for between 2 and 4 years should be between the mortality rates for those who had quit for >4 years and those who quit ≤2 years of the same age,11 that is,  We used
We used  (scenario 1) and
(scenario 1) and  (scenario 2) as lower bound and upper bound, respectively.
(scenario 2) as lower bound and upper bound, respectively.
We estimated models with model parameters under four different scenarios with the combination of these scenarios: A-1, A-2, B-1 and B-2.
Nine transition probabilities ( and
and  ) are from model B. The remaining nine transition probabilities (
) are from model B. The remaining nine transition probabilities ( ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  and
and  ) can be estimated based on model C assumptions. Details of transition probability estimation for model C are available in online supplemental appendix A3. We compared estimates from model B with that from model C.
) can be estimated based on model C assumptions. Details of transition probability estimation for model C are available in online supplemental appendix A3. We compared estimates from model B with that from model C.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
At baseline, the average participant age was 75.1 years, with 53% of participants between 65 and 74 years, 34% of participants between 75 and 84 years and 13% of participants 85 years or older (online supplemental table S1). Women comprised 58% of the sample, and white, non-Hispanics 76% of the sample. About 10% participants reported currently smoking, including 6.6% daily smokers and 3.1% occasional smokers. At the follow-up survey, about 8% participants reported currently smoking, including 5.2% daily smokers and 2.7% some days smokers. Men were more likely (about 25% more) to be current smokers than women.
Table 1 presents the estimated transition probabilities for each the four smoking statuses: daily smoking, some days smoking, recent non-smoking and longtime non-smoking. These results provide 2-year quit rates for current smokers and 2-year relapse rates for recent quitters. As an example, for daily smokers aged 65 years, 83.1% (73.0% daily and 10.1% some days) would still smoke at age 67 years and 12.2% would quit; as a comparison, for a cohort of some days smokers aged 65 years, at age 67 years, 75.7% (16.5% daily and 59.2% some days) would still smoke and 19.4% would quit. For recent ex-smokers aged 65 years, at age 67 years, 53.2% (18.4% daily and 34.8% some days) would smoke again (relapse), and 40.5% would still abstain from smoking. These results also provide 2-year mortality by smoking status. Recent ex-smokers had the highest 2-year mortality rates, followed by daily smokers and some days smokers, and longtime non-smokers had the lowest 2-year mortality rates. For example, the 2-year mortality rates among persons aged 65 years were 6.3%, 4.8%, 5.0% and 2.0% for the four groups, respectively.
Transition probabilities between different smoking states at ages 65–95 years
Figure 2 presents percentages of quitting in the remaining lifetime and successful quitting for current smokers, respectively, and percentages of relapse for recent ex-smokers. SEs of estimates are available in online supplemental table S2. For example, among daily smokers aged 65 years, 61.3% (SE=1.5%) would quit in their remaining lifetime, and 31.5% (SE=1.5%) would quit successfully. Among recent ex-smokers aged 65 years, 53.2% (SE=1.6%) would relapse. The probabilities of relapse are the same regardless of how many years ago someone quit because of the model assumption, that is, all relapses were within 2 years of quitting. Although the percentages of quitting in the remaining lifetime were similar between men and women (online supplemental table S3), women were more likely to quit smoking successfully and less likely to relapse than men.
Per cent of quitting in remaining lifetime, successful quitting and relapse by age. Panel 1: per cent of quitting, daily smokers, some days smokers. Panel 2: per cent of successful quitting, daily smokers, some days smokers. Panel 3: per cent of relapse.
Life expectancy at a given age by participants’ baseline smoking status is shown in table 2, with SEs in online supplemental table S4. For example, life expectancy at age 65 years was 20.0 (SE=0.27), 17.2 (SE=0.30), 16.2 (SE=0.29) and 15.9 (SE=0.29) years for longtime non-smokers, recent ex-smokers, some days smokers and daily smokers, respectively. Quitting between 65 and 77 years of age had a significantly (p<0.05) longer life expectancy as compared with current smokers. All non-smokers had a significantly longer life expectancy than did all current smokers between the ages of 65 and 87 years. When examined by gender (online supplemental table S5), the difference in life expectancy between non-smokers and smokers was similar between men and women. However, quitting smoking contributed to slightly greater gains in life expectancy among women than men. Men benefit from quitting up to age 77 years, while women benefit from quitting up to age 87 years.
Life expectancy by baseline smoking status at ages 65 and 95 years
Sensitivity analysis results
Finally, we examined the impact of the model assumption (no relapse among longtime non-smokers) on our estimates by comparing estimates from model B with estimates from model C, which allows relapse for non-smokers with 2+ years’ abstinence (table 3). The model assumption had no or a very small impact on estimation of per cent of quitting in the remaining lifetime, per cent of relapse and life expectancy for longtime non-smokers, as demonstrated by the similar estimates from model B and model C. In most scenarios, the model assumption underestimated per cent of successful quitting slightly, as estimates from model B were about 0.8%–2.7% lower than that from model C, with relative bias from −2.2% to −6.6%; and overestimated life expectancy slightly for current smokers and recent ex-smokers, as estimates from model B were about 0.1–1.0 years higher than that from model C, with relative bias from 0.4% to 5.9%.
Sensitivity analysis—assess the validity of model assumption
Discussion
Summary of findings
This study provides detailed information regarding the dynamics of smoking status as well as the benefit of smoking cessation on life expectancy among US older adults. Not surprisingly, we found that smoking was associated with a significantly reduced life expectancy and that gains in life expectancy could be achieved with quitting up to age 77 years (up to 77 years for men, and up to 87 years for women). These results on the impact of smoking and smoking cessation on life expectancy among older adults are the first reported in the literature and fill critical gaps in smoking cessation research. Yet, in terms of quitting, although approximately two-thirds of smokers aged 65 years will attempt to quit smoking during their remaining lifetime, only about one-third will be able to quit successfully, while about one in every two recent ex-smokers aged 65 years will relapse. Our estimates are consistent with previous analyses of the probability of relapse or successful quitting and the probability of quit attempts.5–7 9 10
As the age pyramid for the US shifts, due to a greater proportion of persons aged 65 years and older, investigators have sought to characterise the range of health trajectories among the elderly.29 Because of the trend of an increasing percentage of persons aged 65 years and over in the US population, the total number of elderly persons who smoke may increase even if the prevalence of smokers is unchanged.3 Understanding the age differences in cessation and relapse rates as well as benefit of quitting is critical, given that smoking patterns and predictors may differ between younger and older populations. According to 2017 NHIS data, recent successful cessation tends to decrease with age and is lowest among adults aged 65 years and older.3 30 The elderly population represent a heterogeneous group, often being categorised into different strata based on chronological and functional age, and the dynamics of smoking may differ between young-old (65–74 years), middle-old (75–84 years) and old-old (85 years and over).31 32 The large sample size of the Medicare HOS data enables us to obtain estimates, with good reliability, at each of the 2-year age intervals from age 65 years to 95 years, and this is one of the strengths of our study.
Methodological considerations
Many previous investigations of smoking cessation and relapse in the US general population relied on questions of previous quitting attempts and years since quitting from large, nationwide, cross-sectional surveys such as the NHIS, NHANES and BRFSS.5–7 9 10 The main weakness of this approach is the inability to calculate per cent of successful quitting and relapse as well as potential recall bias and selective survival bias. Although some studies used cohort data to analyse the dynamics of smoking, the sample sizes of these studies were usually too small to provide reliable estimates.25 33 Furthermore, these cohort studies used either clinical samples or non-representative samples of the general population.
Because lifetime smoking history is not assessed in the data, we could not examine never smokers and former smokers. Instead, we examined recent non-smokers and longtime non-smokers. We proposed a method based on the microsimulation method that constructs a synthetic cohort of participants with the same baseline smoking status by simulating each individual’s future smoking status until death. This method is novel through using a single current smoking question contained in a large, cohort survey of the US general elderly population with a relatively short follow-up. In this study, the smoking status is assessed and the probabilities of changing smoking status were assessed at two time points. The respondents’ previous smoking status was obtained by modelling transition probabilities between different smoking status from baseline and follow-up surveys. We simulated respondents’ future smoking status through a sequence of independent trials based on transition probabilities between different smoking states from multistate models.
All transition probabilities can be estimated by assuming no smoking relapse or initiation for longtime non-smokers, which includes never smokers and former smokers who quit smoking for >2 years. We made this assumption based on: (1) nearly half of older adults had never smoked before and they were very unlikely to start smoking for the first time. The smoking initiation rate decreased with age after 18 years of age.4 34 Data from 2003 to 2010 NHANES indicate that among ever smokers aged 65 years or older, only 0.1% started smoking at age 65 years or later35; (2) relapse rates would be much lower after 2+ years of abstinence. Previous studies uniformly showed that relapse rates decreased with years of abstinence.5 23–26 28 For example, data from 1449 former smokers in California showed that the likelihood of relapse for those with 2+ years of abstinence was about 93% lower than that for those with <2 years of abstinence.26 Additionally, our sensitivity analysis demonstrated that, for most scenarios, the impact on our estimates was small, with the maximum relative bias within ±7%.
Previous estimates of the impact of smoking on life expectancy in general population relied on the assumption that smoking status would be unchanged throughout the remaining lifetime.8 17 18 As a result, these studies would likely overestimate years of life losses to smoking. The present study was able to account for the possible change in smoking statuses during the remaining lifetime when estimating losses in years of life expectancy to smoking and years of life gained after quitting. By doing so, our estimates were less biased. Moreover, with the application of the microsimulation method, this study was able to estimate probability of successful quitting while previous studies were unable to make such calculations.6 7 9
Limitations
First, because this analysis is based on a survey of Medicare beneficiaries who voluntarily enrolled in private Medicare Advantage health plans, the current sample may be younger and healthier than the overall Medicare population.36 Second, the smoking status was based on self-reported data. However, using self-reported smoking status would be unlikely to have a large impact on our conclusions. Even if some participants under-reported their smoking history, it would not overestimate quitting attempts or underestimate relapse because under-reports were at both baseline and follow-up. Furthermore, under-reporting would not overestimate gains in life expectancy after quitting. Third, respondents reported their current smoking status, and the difference between ‘smoke some days’ and ‘not smoke at all’ in question “Do you now smoke?” is not very clear. Fourth, we assumed only a single transition from baseline to follow-up, and transitions were made at the end of the time interval. This assumption can lead to underestimating percentages of quitting and relapse.16 Fifth, our analysis did not examine factors (other than age and gender) that affected quitting and relapse or the reasons for quitting and relapse. This is because many of these factors are time-varying variables. In order to include these variables in the analysis, values of these variables would need to be treated as additional transitional states which would make our model too complicated to estimate.
Conclusions
This study estimated smoking patterns (quitting and relapse) and the benefit of quitting in a traditionally overlooked demographic subgroup.3 31 These estimates are currently unavailable for the US general population of older adults and would enable an understanding of the trajectory and impact of tobacco use. Such information also would help guide the investment of smoking cessation services as the population ages. Future data collection should include a respondent’s number of prior quit attempts and times advised to quit smoking, given that both will influence quit rates and, ultimately, guide resource allocation and risk messaging.15 37 Additionally, further investigations should aim to develop a broader understanding of smoking cessation predictors to identify specific strategies that might work best for the elderly based on specific sociodemographic features or chronic conditions.
Data availability statement
Data may be obtained from a third party and are not publicly available. This study is a secondary data analysis using the Limited Data Set (LDS) of the HOS from the US Centers for Medicare & Medicaid Services (CMS). The dataset contains potentially identifying or sensitive patient information (eg, participants’ zip code, date of birth, date of death, etc). A signed Data Use Agreement (DUA) with CMS is required to obtain LDS data files (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/HOS). In order to request a LDS file, investigators must follow the instructions on this link: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/EPPEpilot-LDSS.
Ethics statements
Patient consent for publication
Ethics approval
This study was reviewed and approved by the Columbia University Medical Center institutional review board (IRB-AAAR4154). Participants gave informed consent to participate in the study before taking part.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HJ was in charge of the conceptualisation, methodology, software, validation and data curation. Both authors (HJ and EL) were involved in the writing, editing and visualisation; both authors read and approved the final manuscript. HJ had full access to all of the data (including statistical reports and tables) in the study and accepts full responsibility for the integrity of the data and the accuracy of the data analysis.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.




