Article Text
Abstract
Objectives To review and analyse evidence regarding costs for retinopathy of prematurity (ROP) screening, lifetime costs and resource use among infants born preterm who develop ROP, and how these costs have developed over time in different regions.
Design Systematic review and meta-analysis
Data sources PubMed and Scopus from inception to 23 June 2021.
Eligibility criteria for selecting studies Included studies presented costs for ROP screening and the lifetime costs (including laser treatment and follow-up costs) and resource use among people who develop ROP. Studies not reporting on cost calculation methods or ROP-specific costs were excluded.
Data extraction and synthesis Two independent reviewers screened for inclusion and extracted data, including items from a published checklist for quality assessment used for bias assessment, summary and random-effects meta-analysis for treatment costs. Included studies were further searched to identify eligible references and citations.
Results In total, 15 studies reported ROP screening costs, and 13 reported lifetime costs (either treatment and/or follow-up costs) for infants with ROP. The range for screening costs (10 studies) was US$5–US$253 per visit, or US$324–US$1072 per screened child (5 studies). Costs for treatment (11 studies) ranged from US$38 to US$6500 per child. Four studies reported healthcare follow-up costs (lifetime costs ranging from US$64 to US$2420, and 10-year costs of US$1695, respectively), and of these, three also reported lifetime costs for blindness (range US$26 686–US$224 295) using secondary cost data. Included papers largely followed the quality assessment checklist items, thus indicating a low risk of bias.
Conclusion The costs of screening for and treating ROP are small compared with the societal costs of resulting blindness. However, little evidence is available for predicting the effects of changes in patient population, screening schedule or ROP treatments.
PROSPERO registration number CRD42020208213.
- health economics
- paediatric ophthalmology
- medical retina
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Original data are available in the reviewed publications, which are all cited. Additional data from the data extraction performed are available on reasonable request from the corresponding author, including author template data collection forms, data extracted from included studies, data used for all analyses, analytic code, and any other materials used in the review.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
PubMed and Scopus were searched systematically.
Since manual search of reference lists and citations of the identified papers did not identify additional studies, the database search had good coverage of the topic of investigation.
The main limitations of this work were the exclusion of grey literature and the lack of analyses of publication bias for the meta-analysis.
Where lack of variance information in included studies hindered meta-analysis, guidance for synthesis in systematic reviews without meta-analyses were followed.
Introduction
Improvements in neonatal care have resulted in increased survival among children born preterm,1 but these infants are at risk of developing preterm-related morbidities such as retinopathy of prematurity (ROP). ROP is characterised by abnormal neurovascular development and, in its worst forms, retinal detachment and blindness.2 Although preventable, ROP is the leading cause of blindness in children worldwide,3 a ranking associated with the survival of infants with extremely low gestational age and birth weight in some parts of the world, and use of unmonitored treatments with 100% oxygen in other regions.2
ROP management and treatment economics are still challenging in many health systems because of screening-associated costs, patient-related costs and medicolegal liability.4 Thus, there is an urgent need for more concerted efforts to guide healthcare providers in how to use resources efficiently, both in developing economies during a phase of improving survival of preterm infants, such as in many parts of Africa,5 and in countries like Sweden with major neonatal morbidities still affecting a large proportion of those who survive.6
Here, we present an overview of costs associated with ROP screening and treatment, examining the evidence related to costs for ROP screening and lifetime costs (including laser treatment and follow-up costs) and resource use among infants born preterm who develop ROP. We also examine the trajectories of these costs over time in different regions in a meta-analysis.
Methods
This work followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (ie, PRISMA),7 with protocol available in PROSPERO (reference CRD42020208213).8
Article search
PubMed and Scopus were searched (online supplemental eTable 1, 23 June 2021) to identify original research on costs for ROP, including full cost or cost increases associated with ROP, without restricting language, publication date or country. Papers were thus included if presenting costs for ROP screening or lifetime costs (including laser treatment and follow-up costs) and resource use among people who develop ROP. Lifetime costs can for example include follow-up healthcare costs but also productivity loss due to blindness or other cost components occurring due to visual impairment later in life. Articles that did not describe the cost calculation method were excluded, as were those not presenting the costs for the group with ROP separately.
Supplemental material
Rayyan QCRI was used for handling duplicates and the selection of studies for inclusion. Two independent reviewers (JH and CL or HG) searched the databases, screened articles for eligibility, extracted data using a prespecified data extraction sheet (online supplemental eTable 2), and handsearched included studies (7 July 2021) to identify eligible references and citations. Conflicting views were resolved by discussion with a third reviewer (CL or HG).
The data extraction sheet included items (online supplemental eTable 2) from a published checklist for quality assessment of economic evaluations9 including a core set of items relevant in assessing the risk of bias in included studies. The 19 checklist items covers design and methods, population and generalisability, as well as ethics and funding, answered as yes or no during the assessment. To aid reading, summary scores indicating the items answered as Yes for each paper were calculated, thus a high summary score indicates that many of the items were covered. Quality of evidence was rated on a scale from 1 to 5 for individual articles, according to: 1=for example, properly powered randomised controlled trials; 2=for example, prospective cohort studies; 3=for example, retrospective cohort studies; 4=case series with or without intervention or cross-sectional study; 5=for example, opinion of respected authorities.10
Analysis
Conventional screening (excluding telemedicine costs), laser treatment and long-term follow-up costs were reported, respectively, accounting for ROP severity and differences over time and between countries. Identified costs were adjusted to 2020 US dollars (US$) using annual exchange rates11 and the Organisation for Economic Co-operation and Development inflation factor.12 After imputation of missing variance based on the percentage variance in studies presenting such information, treatment costs were summarised in a forest plot, by year and subgroups using the World Bank country classification based on gross national income per capita,13 as cost levels can be expected to differ.
Patient and public involvement
This project did not include patient or public involvement in developing the research questions, design, conduct, choice of outcome measures or recruitment.
Results
Of the 503 studies screened after duplicates from the databases were removed, 123 were assessed for eligibility based on full text, and 19 studies were included in the synthesis of results (online supplemental eFigure 1). Reasons for exclusion were absence of data on costs associated with ROP, lack of original data or inclusion of data related only to insurance payments or litigation. No additional studies were identified by a hand search of references and a Scopus search of citations of included studies. An overview of all included studies14–32 is presented in table 1, including references to secondary cost sources.33–39 In total, 15 studies covered screening costs and 13 reported lifetime costs (treatment and/or follow-up costs) for infants who developed ROP.
Overview of studies included in this review
Twelve studies were conducted in high-income economies: seven in the USA, two in Canada and one each in the UK, Netherlands and France. Three studies were conducted in upper-middle-income economies: one each in Peru, Thailand and Brazil. Three studies were conducted in lower-middle-income economies: two in India and one in Iran. One study was conducted in both the United States and Mexico (table 1). All studies reported the economic analyses using either US dollars, euros or local currency. The patient populations in all studies were infants at risk for ROP, although the studies used different inclusion criteria based on gestational age at birth and birth weight. In addition, the ROP definition for stages and treatment criteria varied with the timing of the study and international guidelines for classification at that time.
Risk of bias in included studies
The quality assessment indicated a high overall quality of the included studies (online supplemental eTable 3), with 16 of 19 of them fulfilling at least 16 of the assessed criteria. However, eight studies did not fulfil the criteria for discounting future costs and outcomes or for subjecting results to sensitivity analyses to address the effects of assumptions. In addition, 14 studies met criteria regarding the reporting of incremental analysis and potential conflicts of interest. Thus, overall, the assessment suggested a low risk of bias in the included papers, and also indicated where lack of reporting on potential conflicts of interest was most problematic. Quality of evidence ranged from 1 to 5 for individual articles, with articles most commonly based on data from retrospective cohort studies (evidence rating 3; nine publications).
Costs for ROP screening
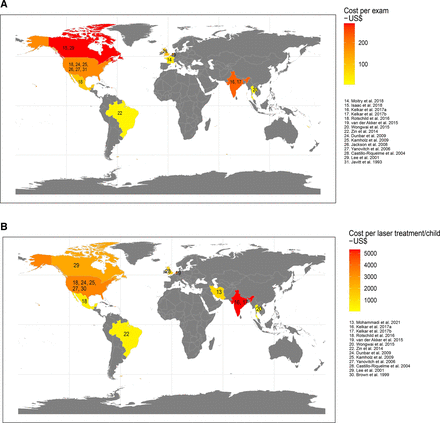
Studies reporting costs related to screening had different designs: six were retrospective cohort studies using medical chart review or register data,15 16 20 24 28 30 nine developed economic models19 21 23 25–27 29 31 32 and two were public intervention studies related to the introduction of ROP screening programmes.17 18 Although the assessment indicated a low risk of bias, screening costs differed substantially among reporting countries (figure 1A).
Map of data availability and costs per (A) screening visit and (B) treatment. The map illustrates reported costs or means of reported costs per country for included studies in US$. In studies presenting only total screening cost per infant or by first/follow-up visits,19 28 32 the cost level per screening was calculated under the assumption of four screening visits per infant. Where only screening cost per eye was reported,30 it was duplicated to obtain the cost level per screening. In studies reporting only unit cost per treatment,20 26 the unit cost was assumed to indicate the cost level of treatment per infant. where costs were reported separately for unilateral and bilateral treatment,29 a weighted mean cost was calculated assuming that 75% of treatments were bilateral.
Costs for routine ROP screening, excluding transportation costs, are reported in table 2. Ten studies reported a mean unit cost per screening of US$137 (range: 5–253). In addition, five studies reported a mean cost per screened child of US$553 (range: US$324–US$1072). Of these, two studies reported comparably low costs21 23 for staff and equipment, whereas Rothschild et al19 reported comparably higher costs in the US setting. One study also included transportation costs,15 and when these costs were removed, screening cost was comparably low. The other studies reported similar costs for screening per child (range: US$324–$602).25 28 29
Costs for screening for ROP among preterm infants (in 2020 values)
Javitt et al32 reported a mean unit cost of US$183 for a first screening and of US$149 for follow-up screening, whereas Lee et al30 reported a mean unit cost of US$112 for screening one eye. Finally, two studies from India17 18 reported screening costs of US$1003 and US$630, respectively, for identifying one child with ROP.
In studies comparing alternative screening or treatment options, no common comparator was identified. The incremental cost reported in Black et al22 indicated a savings associated with higher gestational age at birth (table 1). Jackson et al27 used economic modelling to estimate the cost-utility of ROP screening using telemedicine versus conventional ROP screening. Javitt et al32 used modelling to compare weekly, biweekly or monthly screening.
Costs for ROP treatment
In all, 14 studies reported costs related to the laser treatment of ROP (figure 1B). Four studies of treatment costs were retrospective cohort studies,20 24 28 30 eight were modelling studies14 19 21 23 25 26 29 31 and two were public intervention studies.17 18 In addition, two of the included studies31 32 reported costs for cryotherapy (not included in the analyses below).
Eleven studies reported total treatment costs per child, at a mean US$2442 (range: US$38–US$6500). Castillo-Riquelme et al29 found unilateral treatment costs up to US$1165 and bilateral treatment costs up to US$1514, based partially on secondary data from Brown et al.31 Two studies20 26 cited unit costs of laser treatment of US$4065 and US$5661, respectively. Laser treatment costs are reported in table 2. Dave et al24 described costs for screening and treatment combined (US$2962) in a cohort of children with blindness.
Accounting for the low assessed risk of bias but large expected variation based on cost-levels of individual countries, the meta-analysis by country classification (figures 2 and 3) estimated the average costs in high-income economies to US$2960 (95% CI US$2003 to US$3917). Corresponding figures were US$329 (95% CI US$9 to US$649) in upper-middle-income economies and US$3692 (95% CI US$670 to US$6715) in lower-middle–income economies, respectively. Most studies did not report variance of results, making publication bias analysis unfeasible. However, model diagnostics (I2 and Cochrane Q) indicated high heterogeneity between studies within each country classification, which suggests that the results from the meta-analysis should be interpreted with caution.
Forest plot of treatment costs, by country categorisation. In parentheses, ER of included studies: 1=for example, properly powered randomised controlled trials; 2=for example, prospective cohort studies; 3=for example, retrospective cohort studies; 4=case series with or without intervention or cross-sectional study; 5=for example, opinion of respected authorities. Country abbreviated according to ISO code. ER, evidence rating; REML, restricted maximum likelihood.
Forest plot of treatment costs, cumulative results by year and country categorisation. in parentheses, ER of included studies: 1=for example, properly powered randomised controlled trials; 2=for example, prospective cohort studies; 3=for example, retrospective cohort studies; 4=case series with or without intervention or cross-sectional study; 5=for example, opinion of respected authorities. Country abbreviated according to ISO code. ER, evidence rating; REML, restricted maximum likelihood.
Follow-up costs and resource use among infants born preterm and developing ROP
Only four studies reported follow-up costs occurring after screening and treatment, and although the risk of bias was assessed as low, the reported results largely differed between studies. Castillo-Riquelme et al29 reported healthcare follow-up costs over 10 years of up to US$1695. Dave et al24 reported a lifetime follow-up visit cost of US$64 and a blindness cost of US$146 952. Rothschild et al19 reported lifetime follow-up healthcare costs of US$1681 (USA) and US$2420 (Mexico), whereas the costs for blindness were estimated to be US$92 460 (USA) and US$26 686 (Mexico). Wongwai et al21 reported the lifetime costs of blindness to be US$224 295. In addition, Black et al22 reported the costs per quality-adjusted life-year (QALY) associated with ROP and other comorbidities associated with being born preterm.
Discussion
The studies we identified could be grouped by whether they reported costs for screening, costs for treatment or costs (and QALYs) during long-term follow-up or even from a lifetime perspective. The cost range per ROP screening was US$5–US$253 per visit, or US$324–US$1072 per screened child. Costs for ROP treatment ranged from US$38–US$6500 per child. In addition, four studies reported healthcare follow-up costs, and three reported lifetime costs using secondary data on costs for blindness. Although quality assessment indicated a low risk of bias, comparisons between studies were challenging because of the lack of detailed cost and resource use data.
To our knowledge, this is the first systematic review of ROP costs. Included papers largely followed the quality assessment checklist items of a commonly used tool,40 thus indicating a low risk of bias. However, few of the included articles reported disaggregated cost and resource use data or detailed the included cost components, as is recommended for economic evaluations.41 The main limitations of this work were the exclusion of grey literature and the lack of analyses of publication bias for the meta-analysis. Guidance for reliability in systematic reviews of retinal disorder interventions42 was fulfilled, but the standards for systematic reviews of costs and cost-effectiveness studies were not due to the lack of grey literature assessment.43 Also, since costs were reported purely in a descriptive manner no sensitivity analyses were conducted for alternative categorisations of cost components or country classifications. While not a limitation specific to this analysis but rather of the lack of variance information in the included papers, the findings from the meta-analysis of treatment costs needs to be interpreted with caution after variance was imputed. This lack of variance information also made meta-analysis of screening costs unattainable, since no basis for imputation was available. Moreover, the search strategy and databases are expected to cover largely English-language literature was limited to only two databases, but the reference and citation search yielded no additional studies to include. Thus, we expect our findings to represent a good overview of the available evidence, and that regardless the reservations associated with the meta-analysis to represent current knowledge about costs related to screening and treatment of ROP.
Cost components for ROP screening included staff salaries/time, equipment and maintenance, supplies and staff training. Screening costs for ROP were low compared with other associated costs and, with few exceptions, of the same order of magnitude in the included studies. Exceptions were probably attributable to salary differences.
Screening access and schedules vary between countries.44 With the possible exception of Javitt et al,32 the included studies provided little evidence for how casemix and alternative screening schedules affect costs for screening. Savings are expected, however, and a modelling study using published cost data calculated an annual cost savings from reduced screening of US$3 million in the USA.45 However, with low screening costs, the main benefit is reduced discomfort for the infants and reduced travel costs, which can be substantial.15 The most considerable potential for savings on screening is probably increasing gestational age. US data indicate that ROP frequency increased over time, particularly in infants born very preterm,46 and infants of lower gestational age usually both require more screening visits and have more severe ROP.47 Potential savings have been reported from screening using telemedicine (compared with transporting infants to a specialised hospital),15 or using bedside screening with mobile equipment instead of moving the infants to a specific screening facility48; however, this review did not consider these aspects.
Treatment costs were low compared with the costs for follow-up, with Brazil, Mexico and Peru having substantially lower treatment costs than the other countries. Both Javitt et al32 and Brown et al31 reported low costs for the historically used cryo treatment, at approximately 63% of that for laser treatment. For laser treatment, the cost range was US$2304–$6864 per treated child. None of the studies included the more recent antivascular endothelial growth factor (VEGF) therapy. Moreover, no study reported costs based on ROP stages, age of treated infants, or plus disease status.49 Thus, studies provide little guidance on how treatment costs will develop over time as more infants of lower gestational age survive.
Variation among studies in whether one or two eyes were treated made comparisons less relevant, which may reflect the unilateral schedule used in the historically influential Cryo-ROP study.50 However, Swedish registers indicate that bilateral treatment is common (76% of initial treatments and 97% overall)47 and that retreatment is more frequent among infants with very low gestational age51 and those treated exclusively with anti-VEGF.47
When examining ROP treatment, cost components included staff salaries/time, equipment and maintenance, supplies and staff training. Sometimes anaesthesia costs were reported separately or excluded. Transportation was also a considerable cost component in relation to treatment.20 Other potential costs that were not measured include those for the added time spent in hospital or intensive care, including parental leave, during treatment. Many studies reported only total charges, which are expected to be higher than costs to the healthcare provider. However, use of charges as opposed to costs was not an obvious cause of variation here. Two studies from India17 18 reported high costs compared with other studies of both costs and charges, possibly because of some transportation costs remaining as part of additional components. Thus, the apparent decrease in costs over time in the lower-middle-income economies seen in the meta-analysis should be interpreted with caution.
Although ROP results in high costs throughout life, this outcome is primarily based on secondary data for blindness. As the leading cause of preventable childhood blindness52 and probably the leading cause of childhood blindness in middle-income countries,53 ROP should be associated with much of the estimated costs of blindness. Moreover, it has been argued that costs for blindness do not differ by cause.54 Little evidence was available on follow-up after successful, or partially successful, treatment of ROP. Dave et al24 indicated three healthcare visits over the first 7 years of life, whereas Castillo-Riquelme et al29 did not differentiate visits based on treatment or ROP stage. Rothschild et al included transportation costs, white canes, Braille equipment and supplies,19 but disregarded other costs among children retaining sight. Thus, although costs differ by the severity of visual impairment,55 studies of ROP costs do not tend to report this more detailed level of sight. The current knowledge does not inform potential savings or inform subsidy decisions for ROP treatment developments that can save a little more sight. Taken together, the short follow-up underestimates the total impact of blindness,56 and not accounting for visual impairment results in underestimating the financial impact of ROP.
There is a need for comprehensive knowledge about the costs of ROP, both during the introduction of new ROP screening programmes and in countries with established programmes that are now redistributing resources to handle the increasing survival of very preterm infants with high disease burden. In addition to relevant cost components of ROP (online supplemental eFigure 2), complementary studies of the benefits of various neonatal preventative strategies, including oxygen delivery, are warranted because evidence of the costs resulting from conditions such as bronchopulmonary dysplasia is also lacking.57 Such studies should follow state-of-the-art methods for conduct and reporting of health economic studies.
Conclusions
Although costs of screening and treating ROP are substantial for health systems, they are small compared with the follow-up costs to society of resulting blindness. However, little evidence is available to support predictions about the consequences of changes in the patient population, screening schedule or treatment regimens for ROP.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Original data are available in the reviewed publications, which are all cited. Additional data from the data extraction performed are available on reasonable request from the corresponding author, including author template data collection forms, data extracted from included studies, data used for all analyses, analytic code, and any other materials used in the review.
Ethics statements
Patient consent for publication
Acknowledgments
Thanks to SF Edit, a professional scientific-editing service, for language editing.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors contributed to the design of the study. HG, JH and CL designed the database search and data extraction methods. JH and CL undertook the literature search, assessed studies for eligibility, and extracted data. In case of disagreement, assessments were made in discussion with HG. AH contributed clinical expertise on preterm infants and morbidity. HG, JH, US and CL discussed the data and interpreted the results. HG, JH and CL drafted the manuscript. All authors critically reviewed and approved the final manuscript. HG is the guarantor of the study and thus had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding HG was financed by the Swedish Research Council (#2016-01131). JH was financed by the University of Gothenburg Centre for Person-Centred Care (GPCC) teaching assistant program. US was supported by The De Blindas Vänner. AH was supported by The Wallenberg Clinical Scholars, The Swedish Research Council (#2020-01092), the Gothenburg County Council (ALF project, #426531), the Gothenburg Medical Society, and De Blindas Vänner. CL was supported by The Wallenberg Clinical Scholars.
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests HG is employed part-time by IQVIA, which is a contract research organisation that performs commissioned pharmacoepidemiological studies. Thus, its employees have been and currently are working in collaboration with several pharmaceutical companies. JH reports no competing interests. AH holds stock/stock options in Premalux AB and has received consulting fees from Takeda Inc. CL holds stocks in Premalux AB.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.