Article Text
Abstract
Introduction Non-valvular atrial fibrillation (NVAF) is a high-risk factor for ischaemic stroke. The 2016 European Society of Cardiology Atrial Fibrillation Management guidelines recommend oral anticoagulants (OACs) to prevent stroke in men with CHA2DS2-VASc scores ≥2 and women ≥3. However, in patients with a high risk of stroke and a high risk of bleeding (HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly) score≥3), OAC had a higher risk of bleeding. Left atrial appendage closure (LAAC) is non-inferior to OAC as a means of preventing stroke in several studies. As a minimally invasive intervention to prevent stroke, transthoracic LAAC (TS-LAAC) has a high successful closure rate, but there is a lack of literature reports directly comparing it with OAC. Our research compares TS-LAAC with novel oral anticoagulants (NOACs) and provides an appropriate programme for stroke prevention in a specific population.
Methods and analysis This is a non-randomised controlled trial study protocol, and we will conduct this study from April 2022 to April 2025. The study included 186 patients with confirmed NVAF, 93 of whom completed thoracoscopic LAAC, and the control group treated with NOACs. The primary outcome was the incidence of stroke and systemic embolism, as well as the composite endpoint events (stroke, systemic embolism, myocardial infarction, bleeding, cardiovascular death, etc). Secondary outcomes were ischaemic stroke, haemorrhagic stroke, any bleeding events, death from cardiovascular causes, death from all causes, residual root rate in the surgery group, device-related thrombosis in the surgery group, changes in blood pressure, cardiac chamber size changes, etc. Each subject completed at least 1 year of follow-up.
Ethics and dissemination The study has been approved by the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University, China (approval number: KY2022-013-02). The results from this study will be disseminated through manuscript publications and national/international conferences.
Trial registration number ChiCTR2200058109.
- Stroke
- Adult cardiology
- Pacing & electrophysiology
- Cardiac surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A rare study of direct comparison between thoracoscopic left atrial appendage closure surgery and anticoagulant drugs.
Some outcome measures could provide data support for future study designs.
Use of new but robust surgical techniques.
No randomisation of treatment allocation.
Relatively small sample sizes and single-centre study.
Introduction
Background and rationale
As a common arrhythmia, the most serious complication of atrial fibrillation is thrombosis caused by haemodynamic changes and further thrombus shedding, which leads to ischaemic stroke. Non-valvular atrial fibrillation (NVAF) has become one of the main causes of ischaemic stroke.1 2 Cerebral embolism accounts for 13%–26% of ischaemic strokes, and the proportion increases with age.3–5 The 2016 European Society of Cardiology (ESC) Atrial Fibrillation Management guidelines point out that the risk of thromboembolic events is significantly increased when the CHA2DS2 -VASc score is ≥2 in men and ≥3 in women. Long-term anticoagulant therapy is recommended (Ⅰ, A).6 The risk of ischaemic stroke can be significantly reduced with appropriate oral anticoagulation in patients with atrial fibrillation who do not wish to undergo surgery.7 However, as a vitamin K-dependent antagonist, warfarin has a narrow safety margin for anticoagulation, with a fivefold increased bleeding risk compared with non-anticoagulated patients.8 Although the use of novel oral anticoagulants (NOACs) for antithrombotic therapy is effective and significantly reduces the overall risk of bleeding compared with warfarin,9 there is still an increase in adverse events such as thrombosis and bleeding due to missed doses and inadequate anticoagulation management.10–13 We have observed in large randomised controlled trials (RCTs), such as ARISTOLE14,ROCKET-AF15 and RE-LY16,that the annual incidence of major bleeding events among subjects receiving novel OACs or warfarin ranged from 2.13% to 3.6%, and the cumulative incidence of annual bleeding events (including major and minor bleeding events) ranged from 14.4% to 25.6%. In addition, the discontinuation rate of subjects was as high as 16.6%–25.3% due to bleeding or fear of bleeding risk. When the HAS-BLED(Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (> 65 years), Drugs/alcohol concomitantly) score is higher than the CHA2DS2 -VASc score of the patient, that is, the risk of bleeding is greater than the potential benefit of anticoagulation,17 OACs no longer benefit such patients. In addition, even if novel OACs are taken in patients with previous stroke, the risk of stroke recurrence is still high, up to 9.3%, and the incidence of recent bleeding events, including haemorrhagic transformation, is approximately 7.8% or even up to half.18 Therefore, we need to find a safer and more effective alternative.
Ninety per cent of thrombi in NVAF originate from the left atrial appendage (LAA).19–21 The isolation of the LAA, theoretically, can significantly reduce the occurrence of stroke caused by thrombosis of the LAA from an anatomical point of view. LAA closure (LAAC) as a stroke prevention measure, compared with oral anticoagulation therapy, is non-inferior to warfarin in the risk–benefit prevention of stroke, systemic embolism and cardiovascular death through numerous clinical trials.22–25 In a recent RCT study, PRAGUE-17, LAAC surgery was non-inferior to NOACs.26 The 2016 ESC recommends closing the LAA to prevent thromboembolic events, a level II evidence level b recommendation.6 However, due to the direct contact between the metal mesh of the LAA occlusion and the blood, antithrombotic therapy should not be stopped until it is completely endothelialised to avoid instrument-related thrombosis. Patients implanted with LAA occlusion are advised to receive aspirin combined with warfarin for at least 45 days and continue antiplatelet therapy with aspirin and clopidogrel for 6 months after confirming that there is no thrombus by transoesophageal echocardiography (TOE) examination and then maintain single antiplatelet therapy for a long time.27 Therefore, this strategy does not apply to patients with anticoagulant taboos or patients with a high risk of bleeding. Studies have found that transcatheter LAA occlusion has been shown to have residual leaks in 32% of patients, and transoesophageal ultrasonography in half of the patients revealed LAA thrombus in 22%.28 29 In addition, the incidence of thrombus associated with the use of an endocardial closure device was as high as 7.2%.30 Surgical closure of the LAA also plays an important role in stroke prevention.31 Due to the lack of exogenous foreign bodies in epicardial clamps, the risk of device-related thrombosis is lower in theory. Toale et al systematically reviewed the PubMed, EMBASE and Cochrane Library databases.32 Nine hundred two patients (97.8%) achieved complete closure of the LAA. The success rates of placement under video-assisted thoracoscopy and open surgery were 95.3% and 99.2%, respectively, and no residual leakage was reported. Surgical LAAC has exerted its advantages in preventing stroke through data from the clinical RCT LAAOS-LAAOS III.33–35 However, the comparison with other surgical procedures may disrupt the balance of comparison with OACs alone. At present, there is a new type of LAAC surgery, and the use of video-assisted or total thoracoscopic surgery for LAAC36–39 can be used as a separate operation to prevent stroke. The results of multiple studies have demonstrated high rates of complete closure and low rates of cerebrovascular events during follow-up.40–42
However, there is no direct head-to-head study of oral anticoagulation and surgical LAAC. We designed a non-RCT comparing thoracoscopic LAAC surgery versus concurrent NOACs for stroke prevention in patients with NVAF at high risk of bleeding and stroke to increase evidence for thoracoscopy LAAC surgery as a means of stroke prevention.
Methods and analysis
Study design
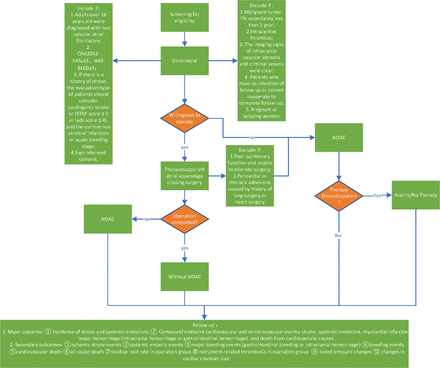
This study is a planned 3-year clinical trial of stroke prevention in atrial fibrillation, comparing the effect of thoracoscopic LAAC surgery with NOACs in stroke prevention. This is a non-randomised controlled clinical trial study. The patients were divided into the intervention group and the control group according to their willingness to accept the intervention measures of thoracoscopic LAAC surgery. In the intervention group, the LAA was clipped with a thoracoscopic LAAC device; the control group adjusted anticoagulation therapy according to the current CHA2DS2-VASc and HAS-BLED conditions. The purpose was to verify the safety and efficacy of thoracoscopic LAAC surgery or its non-inferiority. The trial was approved by the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (ethics number: KY2022-013-02) on 2 March 2022 and registered as the first edition with the China Clinical Trial Center (ChiCTR2200058109) on 30 March 2022. Recruitment will commence from 1 April 2022 to 1 April 2025 in neurology and cardiac centre wards and outpatient clinics. The study flow chart is shown in figure 1.
Study flow chart with inclusion and exclusion criteria, as well as outcome measures. NOAC, novel oral anticoagulant; STAF, Score for the Targeting of Atrial Fibrillation; LADS, the scoring scales for left atrial diameter, age, diagnosis of stroke, and smoking status
Study setting
Data collection will be performed in the Cardiovascular Center and Neurology Department of Beijing Tiantan Hospital, Capital Medical University, Fengtai District, Beijing. The choice of location assignment was not random but was based on data such as the incidence of cardioembolic stroke in patients who had previously visited our hospital.
Eligibility criteria
Inclusion criteria
Adults over 18 years old, diagnosed with NVAF (CHA2DS2-VASc ≥3, HAS-BLED ≥3); if there is a history of stroke or considered cardiac stroke (Score for the Targeting of Atrial Fibrillation (STAF) score ≥5 points or the scoring scales for left atrial diameter, age, diagnosis of stroke, and smoking status (LADS) score ≥4 points), and there is no acute cerebral infarction or bleeding; signed informed consent form.
Exclusion criteria
Malignant tumour; life expectancy less than 1 year; intracardiac thrombus; clear imaging signs such as severe carotid atherosclerosis, intracranial vascular stenosis and criminal blood vessels; presence of patent foramen ovale; other cardiac surgery with thoracotomy; presence of movable aortic plaques (including ascending aorta, aortic arch and descending aortic thoracic segment); patients with no willingness to follow up or who cannot cooperate with completion of follow-up; pregnant or lactating women. Those in the surgical intervention group with poor lung function and unable to tolerate surgery can be transferred to the control group. Those in the surgical intervention group with pericardial or thoracic adhesions caused by the history of lung surgery or cardiac surgery can be transferred to the control group.
Discontinuation criteria
The patient had serious adverse reactions to surgery or anticoagulant drugs.
Interventions
Surgical intervention group (thoracoscopic LAAC)
All patients underwent selective right-sided one-lung ventilation with double-lumen endotracheal intubation under general anaesthesia. Intraoperative TEE was performed and guided intraoperative LAAC. The patient was placed in the right lateral decubitus position with the left forearm raised in line with the shoulder to expose the axillary region. Surgery was performed through three incisions in the chest wall. The camera port (5 mm) is located in the sixth intercostal space of the midaxillary line, and the remaining two working ports (5 mm and 5 mm) are located in the fourth intercostal space of the midaxillary line in front of the axilla and the fifth intercostal space in the posterior axillary line in the rear of the axilla, respectively. The pericardium opens parallel to 2 cm to the right of the phrenic nerve. The pericardium is suspended by a prolene wire and fixed to the skin to fully expose the LAA. The E-clip, the LAAC system of Beijing Med-Zenith, was used to close the base of the LAA in parallel through the 3 cm opening of the sixth intercostal space in the posterior axillary line. Transoesophageal echocardiography confirmed no residual root or residual leakage. After haemostasis and nerve block around the incision, the operation was completed.
Complications during hospitalisation (whether severe bleeding, need for reoperation, myocardial infarction, etc) were observed after surgery, and the complete state of postoperative clipping was reassessed after surgery. According to the clipping state, the following are recommended: (1) incomplete clipping: if there is a residual fistula or the residual root is larger than 1 cm, anticoagulation therapy should be continued; (2) if the clip is complete, anticoagulant drugs should be stopped.
Control group (NOAC anticoagulation group)
All patients in the control group were treated with standard doses of novel OACs (dabigatran 150/110 mg two times per day or rivaroxaban 20/15 mg every day, etc), with appropriate adjustments. If severe bleeding or intolerance occurs during drug administration, discontinue anticoagulant or standard-dose antiplatelet therapy (aspirin 100 mg orally once a day, or clopidogrel 75 mg orally once a day).
Imaging examination and laboratory examination, physical examination and questionnaire survey
ECG or 24-hour Holter ECG examination
Atrial fibrillation rhythm needs to be identified before enrolment, through ECG or 24-hour Holter ECG or long-term ECG (any type, including initial atrial fibrillation, paroxysmal atrial fibrillation, persistent atrial fibrillation, permanent atrial fibrillation, etc); static ECGs were scheduled at the 3rd, 6th, and 12th months after enrolment and during the follow-up.
Cardiac Doppler ultrasonography
Cardiac Doppler ultrasonography was performed before enrolment to determine whether there was thrombosis in the cardiac cavity and whether there was a serious valvular disease; cardiac function (cardiac ejection fraction, cardiac chamber size, atrial size (maximum systolic diameter), left ventricular end-diastolic volume or internal diameter) and intracardiac thrombosis were evaluated at the time of admission, after operation, and at the 3rd, 6th and 12th months of follow-up.
CT angiography
Before enrolment, atrial CT angiography (CTA) was performed to determine whether there was a thrombus in the cardiac cavity, the shape and size of the LAA, and the size of the left atrium. Aortic CTA was used to determine whether there was severe atherosclerosis and plaque in the aorta and cranial and cerebral vessels above the aortic arch before enrolment. Through CTA examination, the operation group was evaluated for the presence of residual root, residual fistula, intra-atrial thrombosis, and displacement of the clamp at the postoperative and 3-month follow-up nodes.
Chest CT
Before enrolment, it should be determined whether there is pleural adhesion and whether there is aortic plaque.
Pulmonary function tests
Before the operation group was enrolled, the lung volume measurement and pulmonary ventilation function needed to be measured: vital capacity >50% predicted value, forced expiratory volume in 1 s >50% predicted value, residual capacity/total lung capacity ratio >50% predicted value, pulmonary diffusing capacity of carbon monoxide >50% of expected value. For ventilation reserve capacity, the following formula is used: ventilation reserve %=(maximal voluntary ventilation (MVV)−minute ventilation)/MVV×100%>70%.
Transoesophageal echocardiography
Before enrolment, the presence of atrial appendage thrombus and the presence of patent foramen ovale were evaluated.
Brain CT
Brain CT was used to determine whether there were imaging signs of stroke, and brain CT was used to determine the presence of ischaemic foci and/or haemorrhagic foci during the 3-month, 6-month and 12-month follow-ups.
Laboratory examination
Electrolytes, B-type natriuretic peptide (BNP), coagulation function, renin inflammatory response indexes, myocardial enzyme indexes, etc, at the time of enrolment and during the follow-up period.
Physical examination
At the time of enrolment and during the follow-up, physical activity examination, muscle strength, muscle tone, nerve reflex, language expression, etc were used to evaluate whether there were any neurological symptoms of stroke.
Questionnaire
Before entering the group, we collected CHA2DS2-VASc, HAS-BLED, National Institute of Health Stroke Scale (NIHSS), STAF and LADS scores. These tables are shown in online supplemental appendix 1.
Supplemental material
Follow-up
The subjects in the group were followed up every 3 months, 6 months and 12 months after they were discharged from the hospital or began to receive treatment. Stroke-related events were evaluated in the examination or questionnaire completed by the hospital or outpatient clinic at that time. We will establish a network consultation platform to guide patients in medication and symptom consultation. During the follow-up period, subjects can no longer receive clinical trials of other cardiovascular drugs.
Clinical evaluation and analysis
Primary outcome measures
(1) The incidence of stroke and systemic embolism in the two groups; (2) the composite endpoint event rate included stroke, systemic embolism, myocardial infarction, major bleeding (intracranial haemorrhage or gastrointestinal haemorrhage) and death from cardiovascular causes.
Analysis
In patients with NVAF, the ultimate goal of either LAAC surgery or OAC therapy is to prevent cardioembolic stroke from causing stroke or systemic embolism. The advantage of LAAC surgery is to highlight the reduction of 90% of the source of thrombus formation in the LAA, thereby reducing the risk of bleeding from long-term OACs. Since cardiac surgery often causes myocardial damage in the perioperative period, the incidence of myocardial infarction and cardiac death can be monitored at the same time on this basis, and the occurrence of overall cardiac and cerebral events can be known to reflect its safety. According to the clinical manifestations of patients combined with CT or MRI, the occurrence of stroke events was determined, and the type of stroke (ischaemic or haemorrhagic) was determined. Then, the specific follow-up time was recorded as the endpoint. Each case is recorded as 1 (whether it is ischaemic or haemorrhagic stroke, including transient ischaemic attack). There is a total of n cases, divided by the overall base of the single group to obtain the stroke incidence rate; at the same time, the follow-up time of each case is calculated to complete the time-to-event rate calculation.
Secondary outcome measures
(1) Ischaemic stroke event; (2) systemic embolism event; (3) major bleeding event (gastrointestinal haemorrhage or intracranial haemorrhage); (4) bleeding event; (5) cardiovascular cause of death; (6) all-cause death (7) residual root rate in operation group; (8) instrument-related thrombosis in operation group; (9) blood pressure changes; (10) changes in cardiac cavity size.
Analysis
Because the incidence of individual event diagnosis is low and a large amount of data is needed, ischaemic stroke events, non-cerebrovascular embolism events, major bleeding events, summation of major and small bleeding events, cardiovascular death and all-cause death are taken as secondary outcome indicators. Although it is a secondary outcome indicator, it is still the focus of this study. In this study, it was found that which kind of event has a higher incidence will become one of the issues discussed in this study, to guide specific clinical treatment. In addition, the effectiveness and safety of thoracoscopic LAAC surgery are explained by the rate of residual roots and the occurrence of instrument-related thrombus events.
In addition, in a prospective, non-randomised study,43 it was found that when there was no significant difference in baseline systolic blood pressure between the epicardial group and the endocardial group, the systolic blood pressure in the epicardial LAAC group was significantly lower than that in the endocardial LAAC group at 3 months and 1 year. Here, we re-explored and verified indicators such as changes in blood pressure and cardiac cavity size in our secondary outcome indicators. The trial outcome is shown in table 1.
Trial outcome in both groups
Sample size and research plan
There are few studies on the direct comparison between thoracoscopic LAAC surgery and anticoagulation therapy, especially in patients with high risk of stroke and high risk of bleeding. According to the non-inferior efficacy study of LAAC surgery and novel OACs in PRAGUE-17’s randomised clinical trial,44 it is estimated that the incidence of annual compound endpoint events is 13% in novel OACs and 10% in LAAC surgery. Based on the literature data of transepicardial clipping of the LAA under one-group thoracoscopy,42 it is considered that in NVAF, the stroke event can be controlled within 1.5%, and the expected maximum value is no more than 6%. Therefore, the target value of this test is 1.5%, and the non-inferior boundary value is δ=5%. In the case that the statistical significance level is unilateral test α=0.025 and the test efficiency is not less than 80% (1−β), according to the above-mentioned data parameters, statistical assumptions and sample estimation formula, PASS V15.0(NCSS, LLC,USA) is used for sample estimation: the calculated sample size needs at least 93 cases in each group, with a total of 186 cases.
Patient allocation
This study is a non-randomised controlled study, and patients will be grouped by their preference for anticoagulation or surgery. According to the anticoagulation data of our centre, the patients in the anticoagulant drug group are much larger than those in the surgery group. Therefore, patients will be 1:1 matched with CHA2DS2-VASc scores and HAS-BLED scores, gender, and age after grouping to control for bias and calibrate baseline information.
Data collection
Baseline information
The collection of baseline information consists of demographic data including age, sex, weight, etc; smoking, drinking, respiratory diseases, cardiovascular diseases, endocrine diseases, neurological diseases, surgical history, family history, past use of drugs (antihypertensive drugs, anticoagulants, antiarrhythmic drugs, etc); physical examination including heart rate, murmur, blood pressure and muscle strength; questionnaire surveys including CHA2DS2-VASc score, NIHSS score, STAF or LADS score, et; laboratory examination including biochemical and inflammatory factors, coagulation factors, myocardial enzymes, BNP, renin, angiotensin, etc. The craniocerebral signs of CT, the size of the atrium and the index of cardiac function were examined by ultrasound.
Data recording of interventions
Operation group: operation time, perioperative complications, drainage volume, perioperative stroke, bleeding, events of death, repeated laboratory examinations, ultrasound and CT examinations were recorded. Oral drug control group: the type, frequency and dose of drugs used were recorded.
Data records during follow-up
The intervention group and the control group were followed up by telephone or network, outpatient clinic. Their clinical symptoms at 3, 6 and 12 months of follow-up were recorded, and they were informed to complete repeated laboratory and imaging examinations. If there is an outcome indicator, it will be recorded in time.
The time points of data collection are shown in table 2.
Overview of study measurements and time points for data collection
Data collection for baseline and follow-up measures was assigned to different members of the research team. Follow-up outcome indicators at each stage were done by different study team members and entered into paper and electronic databases by the study team members who submitted them for data processing. Executive members for the follow-up will not have access to the electronic database.
Data management
Each data controller will be given an account number stored in an electronic database. Research Electronic Data Capture is a secure web-based software platform. The China Trial Management Public Platform will be used to store all data. All data are stored using anonymised codes. The code with the paper data is kept in a lockable warehouse that can only be accessed by researchers involved in the project. Data will be kept for at least 5 years after the study is completed.
Statistical methods
All data can be recorded in the worksheet of Microsoft Excel. All data analysis was performed by using appropriate statistical software, such as IBM SPSS statistics for Windows, V.25 (IBM Corp) software or R statistical software (R Foundation for statistical computing, Vienna, Austria) or Stata V.14.0 software (Statacorp, College Station, Texas, USA). All data are represented by appropriate features such as the mean, SD, median and percentage. We will use the intention-to-treat approach for all analyses in the general population. Categorical variables and continuous variables were analysed using independent t-tests and Χ2 tests, respectively. We will calculate HRs, 95% CIs and p values for time-to-event analyses using a Cox proportional hazards model with risk factors as covariates. A p value less than 0.05 was considered statistically significant. When the proportion of missing data is less than 5% or greater than 40%, it will not be processed. When 10%–20% of the data are missing, the method of multiple imputation is implemented with SAS V9.4(SAS Institute Inc.,USA) software.
Interim analyses
In the mid-term of the clinical trial, the data of the research team will be counted for the main outcome indicators of the two groups, and the safety and efficacy of the closure of the LAA under thoracoscopy will be evaluated, as well as whether there is a significant difference between the resolution indicators of the control group and the intervention group. When the clinical trial indicated that (1) the safety of thoracoscopic LAAC surgery was poor, (2) there were more serious adverse events in the two groups, and (3) the data between the two groups showed that there was a significant difference, if one of the above situations occurs, the trial will be terminated early according to the decision of the ethics committee.
Ethics and dissemination
Ethics
Informed consent
Participants who are interested and meet the exclusion/inclusion criteria will be provided with written and verbal information about the study. Informed consent was provided by specialised researchers. All participants are given the opportunity to discuss participation with family members or other responsible persons close to the participant. Consent is continually monitored during the research by asking participants if they want to continue at the start and end of each session. The specific content of the informed consent form is in online supplemental appendix 2.
Supplemental material
Protocol amendments
Any protocol amendments will be submitted to the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University for approval. The primary investigator will update the trial registry after amendments have been approved.
Harms
The possibility of harm is low. Closure of the LAA in patients at high risk of stroke and bleeding reduces the chance of LAA thrombosis and reduces the chance of stroke and bleeding from anticoagulant drugs. Since its introduction in 2007 in the first in-person prospective device trial (NCT00567515), epicardial LAAC has been shown to be an effective tool for safe and durable LAA occlusion in cardiac surgery patients.45 Ailawadi et al37 reported favourable short-term safety and durability results in a prospective, non-randomised, multicentre study. In addition, 3-year follow-up data were provided in a trial cohort that documented 100% persistent and complete LAAC by CT imaging.38 In non-surgical patients with atrial fibrillation, the risk of ischaemic stroke can be significantly reduced by appropriate OACs.7 In the FDAMAUDE Database, there are unique reports of adverse events using the lariat device,46 but there are no reports of adverse events caused by thoracoscopic closure of the LAA with the epicardial LAA clip device. However, there are still cardiac rupture or coronary events, depending on the surgeon’s judgement. If participants do experience any side effects or adverse reactions, researchers will monitor them, or rescue them, until symptoms subside. The sponsor, Beijing Tiantan Hospital, Capital Medical University, will bear the cost of treatment and give corresponding financial compensation in accordance with relevant national regulations.
Auditing
During the clinical trial process, any modification to all trial protocols should be reported to the ethics committee and implemented after approval. Any events that occur during the trial that may affect patient safety or the continuation of clinical trials, especially changes in safety, should be reported to the ethics committee. Updates to the investigator’s operating manual should be submitted to the ethics committee. A progress report of the clinical trial and a summary of the clinical results after the clinical trial should be submitted to the ethics committee annually.
Confidentiality
The original medical records will be kept in the hospital. Electronic data will be stored in the clinical trial public management platform, and personal data will be stored in random numbers. Investigators, research authority personnel, ethics committees, monitors, auditors, and drug regulatory authority inspectors may consult the subjects’ original medical records to verify the clinical trial process and data. The above-mentioned personnel are responsible for the confidentiality of patients’ personal information, and violations of disclosure will be punished. Any subject-related identification records will not be used publicly and will be kept confidential. If clinical trial results are released, identifying information will remain confidential. We will make every effort to protect the privacy of personal medical information to the extent permitted by law.
Patient and public involvement
Patients and the public will not be involved in the development of the research question or in the design of the study. Patients will receive oral and written information about this trial; however, they will not be involved in the recruitment and conduct of the study. Besides, the burden of the intervention will be assessed by patients themselves. After signing an informed consent form, the participants will be assessed for eligibility and data collection will begin. Dissemination of the general results (no personal data) will be made on demand.
Dissemination plan
The study protocol is published in this journal. The results from this study will be disseminated through manuscript publications and national/international conferences.
Discussion
The most conventional treatment for NVAF is anticoagulation, which is an I, A recommendation. However, in patients with high bleeding risk, the rate of side effects such as bleeding compromises stroke prevention. LAAC is a stroke prevention measure, and systemic OAC in the prevention of stroke, systemic embolism and cardiovascular death in patients with NVAF, according to a large number of clinical trials, has been shown to be non-inferior to warfarin.22 26 Because stent exposure is in direct contact with blood, even with combined anticoagulation and antiplatelet therapy, the incidence of device-related thrombosis associated with endocardial devices can range from 3% to 7.2%.47–50 Transepicardial closure of the LAA theoretically has a lower risk of device-related thrombosis due to the lack of an intravascular foreign body, and anticoagulants can be discontinued. Caliskan et al31 advocated discontinuation of warfarin or novel anticoagulant therapy 3 months after treatment for LAAC.51 Kurfirst et al39 again advocated continuation of anticoagulation for 3 months after surgery; if the patient was in sinus rhythm, anticoagulation should be discontinued at this time. Continued blood flow to the LAA has been shown to increase the risk of stroke in patients undergoing surgical closure of the LAA. The current consensus is that a LAA stump <10 mm is routinely used as the criterion for success.52 Once intraoperative transoesophageal ultrasound confirms the stable position of the clipping device, and the postoperative residual root is less than 1 cm and/or there is no residual fistula, or the presence of a matte surface due to exposed trabeculae, anticoagulant drugs can be stopped immediately.53 Given the potentially catastrophic nature of cerebrovascular events, a robustly designed study is needed to support discontinuation of OAC after transepicardial closure of the LAA. It is not currently possible to make strong recommendations to support discontinuation of OAC after surgery.54 However, for patients with high risk of bleeding, it is clinically meaningful to stop anticoagulant drugs after complete closure of the LAA through epicardial closure, especially for minimally invasive surgery under thoracoscopy, which is more conducive to accept. Therefore, the setting of this article is necessary on the basis of high-risk stroke and bleeding risk.
An additional finding was that in a prospective, non-randomised study, patients with epicardial closure of the LAA compared with endocardial occlusion had a significant decrease in systolic blood pressure at 3 months and 1 year.43 Although the exact mechanism of this reduction in systemic blood pressure is unclear, the most powerful explanation is the persistent downregulation of the renin–angiotensin–aldosterone system (RAAS) and its interaction with epicardial LAAC, sympathetic nervous system and natriuretic peptide.55 In addition, Lakkireddy et al55 found that after transepicardial LAAC, epinephrine, norepinephrine and aldosterone were significantly downregulated immediately 3 months after surgery. Atrial natriuretic peptide (ANP) and BNP levels increased significantly at 24 hours and returned to baseline after 3 months. Even adiponectin, free fatty acid and glucose metabolism are affected by LAAC surgery. The exact mechanisms of these phenomena are poorly understood. Changes in natriuretic peptides and the autonomic nervous system innervating the LAA led to the effects of downregulation of the RAAS.56–58
LAA surgery plays a prominent role in patients with contraindications to anticoagulation, reducing the chance of haemorrhage and stroke. At the same time, changes in endocrine and blood pressure during treatment make LAAC surgery an advantageous surgery, especially the more acceptable thoracoscopic LAAC. Further clinical evidence is still needed to verify that it is more suitable for subjects at high risk of stroke and bleeding.
It is necessary to design an RCT to theoretically randomise patients with NVAF to thoracoscopic LAAC surgery versus NOACs alone to reduce selection bias. However, in the process of clinical practice, there is no such environment in which randomisation is performed without the knowledge of patients. Instead, patients choose to receive surgery or OAC therapy according to their own wishes. Alternatively, patients are more inclined to surgery because of the confusion caused by multiple bleeding events in the past. In addition, patients who choose to receive OACs without surgery are still a large group, although patients have been informed of the risk of bleeding.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors CY and DX conceptualised the work. CY drafted the manuscript. XH, YC, HM, FL, YL, YY, QH, QY and WX critically revised the work for important intellectual content and have read and approved the manuscript. Members of the team will randomly serve as follow-up staff and data statisticians and data monitors at each stage.
Funding The study was supported by the in-hospital funds of Beijing Tiantan Hospital, Capital Medical University (funding project number: XD2020-2023).
Disclaimer The funding body did not contribute to the design of the study, data collection, analysis, interpretation of data or writing the manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.