Article Text
Abstract
Introduction The COVID-19 pandemic has an excessive impact on residents in long-term care facilities (LTCF), causing high morbidity and mortality. Early detection of presymptomatic and asymptomatic COVID-19 cases supports the timely implementation of effective outbreak control measures but repetitive screening of residents and staff incurs costs and discomfort. Administration of vaccines is key to controlling the pandemic but the robustness and longevity of the antibody response, correlation of neutralising antibodies with commercial antibody assays, and the efficacy of current vaccines for emerging COVID-19 variants require further study. We propose to monitor SARS-CoV-2 in site-specific sewage as an early warning system for COVID-19 in LTCF and to study the immune response of the staff and residents in LTCF to COVID-19 vaccines.
Methods and analysis The study includes two parts: (1) detection and quantification of SARS-CoV-2 in LTCF site-specific sewage samples using a molecular assay followed by notification of Public Health within 24 hours as an early warning system for appropriate outbreak investigation and control measures and cost–benefit analyses of the system and (2) testing for SARS-CoV-2 antibodies among staff and residents in LTCF at various time points before and after COVID-19 vaccination using commercial assays and neutralising antibody testing performed at a reference laboratory.
Ethics and dissemination Ethics approval was obtained from the University of Alberta Health Research Ethics Board with considerations to minimise risk and discomforts for the participants. Early recognition of a COVID-19 case in an LTCF might prevent further transmission in residents and staff. There was no direct benefit identified to the participants of the immunity study. Anticipated dissemination of information includes a summary report to the immunity study participants, sharing of study data with the scientific community through the Canadian COVID-19 Immunity Task Force, and prompt dissemination of study results in meeting abstracts and manuscripts in peer-reviewed journals.
- COVID-19
- health economics
- immunology
- epidemiology
- infection control
- public health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The primary strength is the study of the utility and cost–benefit of long-term care facilities (LTCF) site-specific sewage surveillance of SARS-CoV-2 as an early warning system coupled with rapid public health response in the prevention and control of COVID-19 outbreaks in LTCF.
As a vulnerable population with high morbidity and mortality potential from COVID-19, the staff and residents of LTCF are the ideal cohorts to study immune response to COVID-19 vaccines in the background of exposure and/or infection during COVID-19 outbreaks.
Another novel aspect of the study is that it will compare COVID-19 antibody testing using dry blood spots versus plasma samples and examine SARS-CoV-2 specific antibodies detection by multiple commercial assays and compare those results with neutralising antibody testing at a reference laboratory at various time points up to 18 months after COVID-19 vaccination.
One limitation is that for the immunity study, few blood samples were collected prevaccine because of the accelerated timing of the provincial vaccine rollout to LTCF before the initiation of the study.
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has an enormous impact on health, the global economy, society and quality of life. As of 4 April 2021, more than 130 million confirmed COVID-19 cases including 2.84 million deaths have been reported to the WHO.1 The risk of severe illness with COVID-19 increases with age with the greatest risks among those older than 85 years. The death rate ratio was reported to be 130 and 320 times higher among the age groups of 65–74 and 75–84 years old, respectively, compared with 18–29 years old.2 During the first (1 March–31 August 2020) and second (1 September 2020–15 February 2021) pandemic wave, 79% and 60% of COVID-19 deaths, respectively have occurred in residents of long-term care facilities (LTCF), which offer 24-hour nursing care, and in retirement homes.3 4 As of 26 March 2021, LTCF and retirement residences continued to be the most commonly reported COVID-19 outbreak settings in Canada with 4319 outbreaks, 66 287 COVID-19 cases and 12 372 deaths.5 Enhancing protection of this vulnerable population is the foremost task for government, public health and scientific communities.
Early case identification, rapid infection control measures and contact tracing are keys to decrease transmission of COVID-19 at a population level.6 The same principles are applicable to the prevention and control of COVID-19 outbreaks in any setting. To this end, LTCF in some jurisdictions have opted to perform repeated COVID-19 screening of asymptomatic staff and/or residents for early identification of asymptomatic and/or presymptomatic cases. However, large-scale screening requires enormous infrastructure for sample collection and repetitive testing, which can be uncomfortable for staff and residents and consume significant human and financial resources for diagnostic testing. An alternative approach is to have vigilant symptom screening in staff and residents and timely COVID-19 testing. However, COVID-19 can present with non-specific symptoms and high viral load during a presymptomatic phase and transmission from asymptomatic infections have been described.7–9
An innovative approach is to screen for the presence of SARS-CoV-2 in sewage samples to detect COVID-19 infections. Detection of SARS-CoV-2 in stool samples has been reported in 40%–50% of cases with diarrhoea and 14% without diarrhoea, and diarrhoea reported to be the first manifestation of COVID-19 in 23.3% of cases.10–13 No significant difference was found in SARS-CoV-2 viral loads in stool samples from cases with symptomatic COVID-19 infections and those who were asymptomatic, but a shorter duration of viral shedding was observed in asymptomatic infections in most studies.14 On the other hand, Hoffmann and Alsing have analysed publications on faecal shedding and estimated a mean of 1.9×106 gene copies of SARS-CoV-2 per mL of faeces for hospitalised patients and no evidence that infected individuals do not shed the virus.15 Correlation between the levels of SARS-CoV-2 in sewage samples and disease burden of COVID-19 in the sewershed over time has been reported, but the utility of sewage SARS-CoV-2 in a predictive model for COVID-19 needs to be further investigated.16 17 In contrast, Larson et al published derivative algorithms to identify COVID-19 case zero within a sewershed using SARS-CoV-2 sewage surveillance collected from manholes.18 Betancourt et al have demonstrated the value of sewer surveillance for identifying potential COVID-19 outbreaks on university campuses.19 Since the 1980s, the bylaws of the city of Edmonton have specified that newly constructed buildings need to have a single point of access to the sewage discharge from the building, which provides a unique opportunity to look at site-specific sewage surveillance for SARS-CoV-2 in LTCF.
Another area that requires more study is the immune response to COVID-19 vaccine. In Alberta, both Pfizer-BioNTech (BNT162b2) vaccine (Pfizer, New York, New York, USA) and Moderna (mRNA1237) vaccine (Moderna, Cambridge, Massachusetts, USA) have been administered to staff and residents at LTCF starting mid-December 2020. Preliminary data suggested a faster decline of the level of neutralising antibodies among vaccinees in older adults over 71 years old as compared with younger individuals.20 21 Further studies are required to understand the robustness and longevity of vaccine-induced antibodies in the older age groups. Furthermore, data on the correlation of antibodies detected by commercial assays and neutralising antibody tests are lacking. In addition, understanding the efficacy of currently licensed COVID-19 vaccines against emerging SARS-CoV-2 variants of concern is an urgent task.22–25
To fill these knowledge gaps, our study has two components. Part I of the study will look at the utility and feasibility of LTCF site-specific sewage surveillance of SARS-CoV-2 as an early warning system for COVID-19. This early warning system is coupled with rapid Public Health notification and response to prevent outbreaks in LTCF. A secondary objective here is to perform a cost–benefit analysis of this early warning system to estimate the monetary value of reduced healthcare resource use due to reduced testing and COVID-19 cases in the LTCFs.
Part II of the study entails the measurement of the antibody response to COVID-19 among staff and residents of LTCF, stratified by their exposure to and history of infection with COVID-19 during outbreaks and immunisations. A secondary objective here is to evaluate the performance of multiple commercial assays to detect vaccine-induced antibodies and correlation with neutralising antibody responses.
Methods and analysis
Study design and setting
Both part I and part II of the study are conducted in the setting of LTCF in the city of Edmonton, an urban centre in the province of Alberta, Canada with a regional population of 1.05 million in 2020.26 LTCF in Alberta fall into three categories: public (Alberta Health Services (AHS), the provincial health delivery system provider), voluntary (faith-based) and private (for profit). Voluntary and private LTCF are contracted providers to AHS. In Alberta, continuous masking of all staff and enhanced symptom monitoring of both residents and staff is the strategy adopted by all LTCF to have an early diagnosis of COVID-19 cases so appropriate infection control and contact tracing measures can be implemented. The identification of a single case of COVID-19 among LTCF residents and/or staff with exposure to anyone at the facility is defined as a COVID-19 outbreak as of August 2021. A provincial outbreak investigation protocol (https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-outbreak-management-congregate-guidelines.pdf) was developed to provide guidance for COVID-19 outbreak investigation and management overseen by a Public Health physician (MOH—Medical Officer of Health). Each outbreak is assigned a unique exposure investigation (EI) number with tracking of COVID-19 testing and results.27 28 Samples collected for COVID-19 testing related to outbreak investigations are labelled with patient identifiers and the specific EI number. All outbreak-related testing is performed at the Alberta Precision Laboratories(APL)-Public Health Laboratory. During an outbreak investigation, repeat testing of all residents and staff for COVID-19 at regular intervals (5–7 days) are performed besides enhanced symptom monitoring and testing of any staff or resident with new or worsening symptoms for early detection of new cases. Some LTCF, especially those facing challenges with accurate symptom monitoring of residents, continue to do regular, usually weekly, testing for COVID-19 for all residents after an outbreak was declared over, which is defined as no COVID-19 case for 28 days, that is, two incubation periods for COVID-19. The deployment of COVID-19 vaccination for staff and residents of LTCF was coordinated by AHS Edmonton zone for each facility with vaccines provided by Alberta Health, Government of Alberta.
Patient and public involvement
No patient was involved in the development of this study.
Part I sewage SARS-CoV-2 surveillance as early warning system and public health response
Selection of LTCF
EPCOR Water Services (EPCOR), which manages water distribution and wastewater collection systems in Edmonton, has professional teams that access and collect sewage samples from manholes throughout the city of Edmonton. Ten stand-alone LTCF with various histories of COVID-19 outbreaks and feasible access to site-specific sewage were selected for the sewage surveillance study after review by EPCOR, the lead MOH of Edmonton zone and the Medical Director of Continuing Care, Edmonton zone.
Sewage sample collection and testing for SARS-CoV-2 viral load and public health response
Two times per week, trained personnel of the EPCOR team collect 500 mL of raw sewage sample, either a grab sample or a 24-hour composite sample if weather permits, using the ISCO GLS with 2.5-gallon bottle autosampler (ISCO Industries, Louisville, USA) for each selected LTFC. Composite sample is the preferred sample type as sewage is being collected over 24 hours instead of sampling at only one time point with the grab sample. The planned study duration is 12 months with sewage sample collection and testing started for six sites on 6 January 2021 and additional four sites on a different route on 21 January 2021. Samples are transported in a cooler with ice pack and delivered to the laboratory within 6 hours to be processed for the detection and quantification of SARS-CoV-2. Results are reported within 24 hours from the time of collection.
To test for SARS-CoV-2, sewage samples are processed as previously described with modifications.29 Briefly, 100 mL of sewage sample are adjusted to pH 9.6–10 using 5N NaOH and mixed vigorously for 30 s and then centrifuged at 4500 g for 10 min to pellet solids. The liquid fraction is transferred into a new container and adjusted to pH 7–7.5 with 1.2N HCl. Viruses present in the liquid fraction are concentrated by ultrafiltration using a Centricon Plus-70 filter with a pore size or nominal molecular weight limit of 30 kDa (Merck Millipore, Carrigtwohill, Ireland) according to the manufacturer’s instructions, except for the prerinse step. Filters are loaded with 70 mL of sample and centrifuged at 3000 g for 10 min at room temperature. The filtrate is discarded, and the same procedure is repeated for the rest of the sample. The filtrate collection cup is removed, and the concentration cup is placed on top of the sample filter cup. The device is then inverted carefully and centrifuged at 800 g for 2 min. The sample is collected from the concentration cup and adjusted to a final volume of 1 mL using phospate-buffered saline. Nucleic acid is extracted from 400 µL of the concentrated sample and eluted in 100 µL using MagMAX-96 Viral RNA Isolation Kits on KingFisher Flex Purification System (Thermo Fisher Scientific, Ontario, Canada). A one-step real-time reverse transcription quantitative PCR (real-time RT-qPCR) assay that targets the N1 and N2 regions of SARS-CoV-2 is performed in duplicate on an ABI 7500Fast PCR instrument. An external standard curve is used to quantify SARS-CoV-2 RNA. All amplification curves are reviewed by a research technologist and positive and negative controls of each run need to meet quality control criteria before results are reported. The crossing threshold (Ct) cut-off for a positive RT-qPCR is 40, that is, Ct ≤40 is positive. When at least two of the four results generated by duplicated N1 and N2 tests, for example, both N1 or both N2, or one of N1 and one of N2, are positive, the sewage sample is reported as positive for SARS-CoV-2. When only one of the four results is positive, the sample is reported as indeterminate; when all four RT-qPCR results are negative, the sample is reported as negative. For quality assurance, 5 µL of salmon DNA (Cat # D1626, Sigma, Canada) is spiked into the viral concentrate prior to RNA extraction to check for PCR inhibition. The presence of inhibition is defined as a delay by at least three PCR cycles (Ct value) as compared with a distilled water control spiked with the same amount of salmon DNA. Detection and quantification of Pepper mild mottle virus is used as an endogenous virus-maker indicative of human faeces to normalise quantifiable results of SARS-CoV-2 in the sewage samples to account for variable water dilution. Categories of reported results of SARS-CoV-2 in sewage samples include negative, indeterminate, positive but not quantifiable, and positive. Quantified results are reported as genomic copy numbers per 100 mL of sewage.
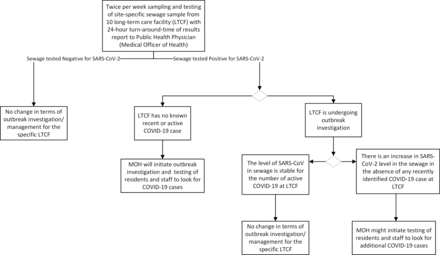
The results of SARS-CoV-2 in sewage from the ten LTCF are reported to both the MOH and Medical Director of Continuing Care, Edmonton zone or their designates within 24 hours from the collection of sewage samples. Depending on the current status of each LTCF, different actions will be coordinated by the MOH (figure 1). For example, detection of SARS-CoV-2 RNA in sewage from an LTCF with no known COVID-19 case or a significant increase in the level SARS-CoV-2 with no newly diagnosed COVID-19 cases, is likely to trigger COVID-19 screening of residents, staffs and visitors with collection of nasopharyngeal or throat swab by the outbreak investigation team according to an existing protocol. Infection Prevention Control measures and contact tracing will be implemented as appropriate with the identification of COVID-19 cases.
Flow diagram of SARS-CoV-2 sewage testing, result reporting and possible public health response. MOH, Medical Officer of Health.
Part II COVID-19 immunity study
Recruitment of staff and residents of LTCF for the vaccine immunity study
An infographic and a letter of invitation was circulated to three professional membership bodies for continuing care providers in Alberta, namely Alberta Continuing Care Association, Alberta Seniors Communities and Housing Association, and the Christian Health Association of Alberta, asking for the information to be distributed to their members to solicit interest in the study. Follow-up meetings were arranged with providers who expressed interest to explain the objectives and design of the study.
Five hundred consenting LTCF staff and residents will be recruited by: (1) face-to-face consent during site visit arranged by study coordinators with the Director of Care or Manager of each LTCF and (2) phone consent with standard scripts obtained from staff, residents and substitute decision-makers of residents who have received a consent form with a cover letter as distributed at each participating site by the providers or study coordinators or by reading posters posted at participating LTCF. Recruitment started on 12 February 2021 and is continued till the target number is reached or up to the last time point of blood collection, that is, 18 months after the participants have received two doses of vaccine.
Collection of blood samples and SARS-CoV-2 antibody testing
Depending on immunisation history and timing of recruitment, blood samples are collected a minimum of one and maximum of four times (table 1). At each time point, one attempt is made to perform a venous blood draw using a 6 mL BD Vacutainer Plastic Blood Collection Tubes with K2EDTA (BD-Canada, Mississauga, Canada). If this attempt is unsuccessful, dried blood spots (DBS) will be collected using Whatman 903 protein saver cards (Cytiva, Mississauga, Canada) following standard DBS collection, storage and shipment protocol with training provided by the National Microbiology Laboratory, Winnipeg, Manitoba, Canada. Only one attempt of venous draw is allowed in the protocol to minimise stress, pain and bruising for participants, especially those with difficult venous access, for whom DBS will be collected. The EDTA blood tubes and DBS are kept at room temperature and transported to the research laboratory within 24 hours. If there is sufficient blood collected in the EDTA tubes, at least two DBS are created using 75 µL of whole blood from the EDTA tube to fill a blood spot from the centre of the circle after gently mixing the blood by inversion. The residual whole blood in the EDTA tube is centrifuged for 10 min at 2500× g to separate the plasma to create 500 µL aliquots. Plasma aliquots and sets of 10 appropriately dried DBS packed with desiccant pouch and humidity indicator in gas impermeable bags are stored at −80°C before testing.
Schedule for blood collection in immunity study as relationship to vaccination
The assays and antigen target for the COVID-19 antibody tests to be performed on plasma samples and DBS are summarised in table 2. The Bio-Rad BioPlex 2200 SARS-CoV-2 IgG Panel (Bio-Rad Laboratories (Canada), Mississauga, Canada) is the only test that will be performed on all three sample types: plasma and DBS prepared from each EDTA whole blood sample, and DBS collected from participants whose venous blood draw was unsuccessful, that is, only one test can be performed when a DBS instead of venous blood is collected.
Testing of plasma and dry blood spots for SARS-CoV-2 specific antibodies
The testing and result reports of the plasma sample using the commercial assays will be performed according to the manufacturers’ instruction. Neutralising antibodies against SARS-CoV-2 are detected on plasma samples using plaque reduction neutralisation test as previously published.30 Sera free of anti-SARS-CoV-2 antibody, for example, anti-SARS-CoV-2-negative sera procured by the Alberta biorepository prior to the COVID-19 pandemic (November 2019), will be used in the assays as negative control. Seropositivity to various antigens will be determined by three out of the five assays performed on the plasma samples. For timepoints where participants only have a DBS collection, seropositivity will be determined by (1) interpretation of the Bio-Rad BioPlex 2200 SARS-CoV-2 IgG Panel and (2) correlation and/or determination of the central tendency of data between the Bio-Rad BioPlex 2200 SARS-CoV-2 IgG Panel and the ARCHITECT SARS-CoV-2 IgG and IgG II assay (Abbott, Illinois, USA) and the SARS-CoV-2 Surrogate Virus Neutralization Test Kit (RUO) (GenScript, Piscataway, New Jersey, USA) from the plasma versus DBS prepared from the same EDTA whole blood sample study.
Data collection
All electronic study data is being kept on the AHS server with access restricted to the study team. Some of the data to be collected for part I and part II of this study are the same for the LTCF with differences summarised in table 3. Facility level data including characteristics of the LTCF and its staff and residents, COVID-19 outbreaks from January 2020 (start of the pandemic in Alberta) to the end of the 18-month study are collected for both LTCF included in the SAR-CoV-2 sewage surveillance study and the immunity study. Participant level data including demographic information, history of COVID-19 infection and vaccination are collected only for the immunity study. All identifiable data will be removed from the analytical database after the data is merged.
Data elements to be collected for part I (SARS-CoV-2 sewage surveillance and early warning and rapid public health response) and part II (COVID-19 immunity study) of the study
A requirement of the study sponsor (Canada’s COVID-19 Immunity Task Force, CITF) is that de-identified data collected as part of the study be deposited into a national database for future research use. This data will include the history of COVID-19 infection, vaccination and results of blood sample analysis. The data on the CITF database will be held in centralised servers outside Alberta, under the custodianship of McGill University or one of its collaborators. The data in the CITF database will be shared with researchers performing for-profit and non-profit research securely via the Cloud to conduct research concerning COVID-19 and related health outcomes. The CITF’s Data Access Committee will require researchers to confirm that their intended research activities have received necessary ethics approvals.
Data analyses
For the SARS-CoV-2 sewage surveillance study, the percentage of sewage samples tested positive for SARS-CoV-2 overtime will be plotted against the rate of newly diagnosed COVID-19 cases among the total number of residents and staff as identified at various LTCF during the study and a regression analysis to be done to generate the sensitivity of the site-specific sewage surveillance system in detecting a new COVID-19 case. Long-term-care facilities without SARS-CoV-2 sewage surveillance with a similar number of residents and level of care will be selected from the city of Edmonton continuing care database as control sites for comparison of the number and the size of the outbreaks with LTCF with sewage-surveillance during the study. Cost-effectiveness of the SARS-CoV-2 sewage surveillance will include the costs of the sewage sampling, testing, reporting, management and targeted public health actions. Patient care costs for testing, clinical care provision including hospitalisation and intensive care unit care will be obtained from the AHS database. Cost–benefit analysis will be done using a return-on-investment method comparing the sewage sample testing technology costs to healthcare and LTCF costs without the early warning system, and incremental cost–utility analysis will provide the cost per the quality adjusted life year estimates.
Antibody test results for plasma aliquots and DBS made from the same whole blood sample (BioPlex 2200 SARS-CoV-2 IgG Panel) will be analysed using the McNemar test for the categorical positive and negative results and linear regression for quantitative antibodies levels for the positive samples. With the estimation that the sensitivity to detect SARS-CoV-2 antibodies by BioPlex 2200 SARS-CoV-2 IgG Panel is 80%–90% and there is minimum 10% difference between positive versus negative test results between plasma and DBS samples, the number of samples needed to achieve 90% power and significant p<0.05 is estimated to be between 245 and 471.31 The proportion of positive and negative and distributive statistics will be used to summarise the results of antibodies to SARS-CoV-2 generated using the various assays and at the different time points for all the participants. Cochran’s Q test will be used to compare the categorical results for plasma samples tested using the four commercial assays. Multiple logistic regression will be used to compare the results of the antibodies in terms of age, staff versus residents, history of COVID-19 infection, and history of COVID-19 exposure for the different assays.
Timeline of the study
Funding approval was announced on 11 November 2020. Part I of the study, SARS-CoV-2 sewage surveillance started in the week of 4 January 2021 with six LTCF sites and increased to ten sites after 2 weeks. Recruitment of staff and residents from LTCF started on 12 February 2021 and is continued to reach 500 participants.
Ethics and dissemination
This study has been approved by the ethics committee of the University of Alberta (approval # Pro00106423). To protect the staff and residents at the LTCF, all the study coordinators received training regarding hand hygiene and donning and doffing of personal protective equipment and follow the fit-for-work screening before visiting LTCF. Each study coordinator only visits one LTCF site per day and no more than three study coordinators will be on site at the same time to minimise risk for the staff and residents of LTCF. Individual results of the COVID-19 antibody tests are not provided to each participant, but the results of the immunity study as a summary report will be shared with the participants.
In addition to the reporting of the SARS-CoV-2 sewage detection to local public health within 24 hours, a scientific advisory committee including the study team members with boarder representation from public health, local and national expertise was formed to have monthly meetings to review progress and findings of the study to enhance real-time knowledge translation. The results of part I and part II of the study will also be shared through the preparation of meeting abstracts and scientific manuscripts through peer-reviewed, open-access journals. Anonymous data will also be shared with scientific community through CITF as described.
A challenge and limitation of the study is the natural progression of the COVID-19 pandemic which peaked during the last 2 weeks in December in Edmonton and the accelerated timing of vaccination for both staff and residents of LTCF in mid-December 2020. With the SARS-CoV-2 sewage surveillance having started at the downturn of the second wave, there are fewer outbreaks; thus, the ability to assess the early warning system is limited. With the immunity study recruitment having started in mid-February 2021, the assessment of antibody levels is limited to primarily postvaccine timelines. However, the study is well situated to study the impact of COVID-19 variants of concerns and the possibility of a third wave of COVID-19 infections in the province.
The assessment of SARS-CoV-2 sewage surveillance as an early warning system for rapid public health response provides a prototype to monitor SARS-CoV-2 in other settings such as dormitory and worker housing facilities as well as provide a model for surveillance of other pathogens that have gastrointestinal or urological pathways and shedding. The immunity study will fill important knowledge gaps about protective immunity in this vulnerable population and allows assessment of different methods of blood collection and various assays for testing for antibodies against SARS-CoV-2.
Ethics statements
Acknowledgments
We thank the staff at EPCOR who assist with the collection and transport of the sewage samples, the Edmonton Zone outbreak investigation team for their work in outbreak investigation and management, the LTCF providers for agreeing to participate in the immunity study and the directors of care and site managers of the various LTCF with their help and support in working with study coordinators, Nancy Ruholl, Sara Moradipoor and Sharmi Biswas, with the recruitment of staff and residents, and last but not least, the staff, residents and substitute decision-makers for providing consent and participation in the immunity study. We also thank the staff of Dr XL Pang’s laboratory for processing and testing the sewage sample and the processing of the whole blood EDTA samples and DBS, staff from APL-Public Health Laboratory for processing and performing the diagnostic testing for COVID-19 outbreaks, AESCO staff for collecting the blood samples at LTCF and Dynalife Laboratory for collecting the blood samples in the community. Many thanks also go to Christine Mesa, Francois Cholette and Philip Lacap from Dr John Kim’s laboratory and to Kristina Dimitrova, Clark Phillipson, Michael Chan from Dr Heidi Wood’s laboratory for their work and support for the immunity study. We specially acknowledge the scientific discussion with Dr James Talbot, Dr Lyndon Gyurek, Dr Jeff Charrois, Dr Rasha Maal-Bared, Dr Norma J Reucker and the contribution from Dr Eloisa Hasing, Dr Sudha Bhavanam, Dr Jiaao Yu, Emma Zwaigenbaum and Melissa Wilson in Dr XL Pang laboratory for the technological development of SARS-CoV-2 testing in sewage samples and the scientific discussion regarding the immunity study with Dr Mike Drebot as well as Dr Bruce Mazer and other CITF research teams.
References
Footnotes
Twitter @KanjiJamil
Contributors X-LP, BEL and CS conceptualised the SARS-CoV-2 sewage surveillance study and developed the initial protocol with critical and scientific contribution from DF, ER, LAL, YQ, TG, RB, SC, SEH, AO and CAE. BEL, X-LP, A-CG, CC, JK and HW designed the immunity study with critical and scientific contribution from AR, JNK, NZ, SFO"B and SD. BEL, X-LP and TG drafted the initial manuscript that was reviewed by all authors.
Funding This study is supported by Public Health Agency of Canada through the COVID-19 Immunity Task Force (RES0052990).
Competing interests SD acts as a content expert for Roche on Arbovirus and respiratory viruses for Johnson and Johnson (Janssen)—companies not involved with this study.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.