Article Text
Abstract
Introduction Recently, the rate of caesarean sections (CS) worldwide has risen and CS-associated complications such as niche have increased substantially. Until now, evidence-based clinical guidelines for the treatment of niche-related symptoms remain absent. In patients with postmenstrual spotting, it has not been studied if the effect of levonorgestrel 52 mg intrauterine system (LNG-IUS 52 mg) is superior to that of hysteroscopy. This study will answer the question of whether LNG-IUS 52 mg is more effective in improving postmenstrual spotting than hysteroscopic niche resection in women with niche-related spotting at 6 months after randomisation.
Methods and analysis This is a randomised controlled trial. A total of 208 women with postmenstrual spotting related to niche in the caesarean uterine scar of at least 2 mm and residual myometrium of at least 2.2 mm evaluated by MRI will be included. Women desiring to conceive within 1 year, with contraindications for LNG-IUS 52 mg or hysteroscopic surgery will be excluded. After informed consent is obtained, eligible women will be randomly allocated to LNG-IUS 52 mg or hysteroscopic niche resection at 1:1. The primary outcome is the efficacy in reducing postmenstrual spotting at 6 months after randomisation. The secondary outcomes include menstrual pattern, total days of blood loss per month, rate of amenorrhoea, side effects and complications.We will use a Visual Analogue Scale for chronic pelvic pain, urological symptoms and women’s satisfaction (five-point Likert scale).
Ethics and dissemination The study was approved by the local medical ethics committee and by the Institutional Review Board of the International Peace Maternity and Child Health Hospital, Shanghai, China (No. GKLW 2019-08). Participants will sign a written informed consent before participation. The results of this study will be submitted to a peer-reviewed journal for publication.
Trial registration number ChiCTR1900025677.
- gynaecology
- surgery
- radiology & imaging
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first randomised controlled trial that will provide evidence for the effectiveness of levonorgestrel intrauterine system 52 mg versus hysteroscopic niche resection in reducing niche-related spotting symptoms.
The trial is adequately based on a representative cohort study.
This trial is an open-label trial both for patients and for researchers, but uses a blind method to evaluate the effect of treatment.
This study will evaluate niches by three-dimensional (3D) MRI with the section of only 1 mm and 3D reconstruction to improve the accuracy.
The trial is based in a single centre, which might limit the generalisability of the findings.
Introduction
In the last decades, the rate of caesarean sections (CS) worldwide has risen and CS-associated complications such as niche have increased substantially.1 In China, in the past 30 years, due to the implementation of the family planning policy, the rate of CS increased from 2% to 62.5% (1985–2014), and in some regions, the rate of CS even reached more than 80%, while not all CSs are medically indicated.2 3 The rapid rise of CS rate has aroused great concern about the poor uterine scar healing and potential long-term complications of CS. Postmenstrual spotting related to a niche in the CS uterine scar is also called a caesarean scar defect (CSD) or isthmocele. The niche was first described by Morris in 1995 and was defined by the ESGE niche task force group as an indentation in the myometrium of at least 2 mm at the site of a previous CS scar.4–6 Niches were reported in 24%–69% of the women with a previous CS who had used transvaginal ultrasonography without intrauterine application of saline or gel, while the rate after application of intrauterine gel or saline ranged between 56% and 78%.7–9 Postmenstrual abnormal uterine bleeding (AUB) is the most common symptom, other symptoms include menorrhagia, chronic pelvic pain and secondary infertility and related adverse effects on women’s physical and mental health. Niches could be associated with pregnancy-related complications such as caesarean scar pregnancy, malplacentation, uterine rupture and massive haemorrhage that consume a large number of medical resources.10
Currently, the treatment of niche-related symptoms includes non-reconstruction of lower uterine segment (LUS) treatment and reconstruction of LUS treatment. Non-reconstructive treatment includes oral contraceptive, levonorgestrel intrauterine system (LNG-IUS), expectant treatment or hysteroscopic treatment. Reconstructive treatment includes laparoscopic repair and vaginal repair.5 11–13 Evidence-based clinical guidelines for the treatment of niche-related symptoms remain absent. The specific treatment method should be determined according to the patient’s symptoms, fertility desire, niche features including size and thickness of the residual myometrium. The evaluation of treatment effect still needs long-term observation and follow-up.6 In recent years, hysteroscopic treatment seems to be a promising option in managing of small niches to reduce postmenstrual spotting.11 12 14–17 Resecting the distal rim aims to facilitate the outflow of menstrual blood and coagulating the surface of vessels in the niche concurrently aims to reduce blood loss from these fragile vessels. A randomised controlled trial compared hysteroscopic niche resection with expectant management in 100 women.The median postmenstrual spotting period reduced by 4 days from the baseline and by 3 days from the control group.12 Both studies reported low complication rates that were prospectively evaluated.11 12 LNG-IUS 52 mg is a long-acting IUS, which has been listed in more than 100 countries after its registration in Europe in 1990.18 Millions of women received an LNG-IUS 52 mg worldwide.19 LNG-IUS 52 mg has been shown to reduce menstrual blood loss and dysmenorrhea through the suppressive action of levonorgestrel on the endometrium proliferation. Even though LNG-IUS 52 mg has been proven to be an effective means of reducing AUB, and AUB is one mean point in niche-related symptoms, evidence that LNG-IUS 52 mg can alleviate the niche-related symptoms remains limited.20 21 Concerning LNG-IUS 52 mg, a retrospective study of Chen et al including six women indicated a positive effect, while Zhang et al found that LNG-IUS 52 mg has little improvement on shortening postmenstrual spotting based on five patients with ING-IUS 52 mg.22 23 Until now, it is unknown which therapy is most beneficial for patients with niche-related symptoms due to the lack of comparative studies.
Objective and hypothesis
The objective of the trial is to determine whether LNG-IUS 52 mg is more effective than a hysteroscopic niche resection in reducing postmenstrual spotting in women with a relatively small niche (residual myometrium ≥2.2 mm) and without a desire to conceive within 1 year. Our hypothesis is that in women with a relatively small niche (residual myometrium ≥2.2 mm) and without a desire to conceive within 1 year, LNG-IUS 52 mg will decrease postmenstrual spotting better than hysteroscopic niche resection.
Methods and analysis
Study design and setting
The study is a single-centre randomised controlled superiority trial and will be performed in the International Peace Maternity and Child Health Hospital, Shanghai, China.
Participants
Inclusion criteria are as follows:
Age 18–48 years old.
Postmenstrual spotting after CS.
MRI has shown: (1) a niche in the anterior wall of LUS of at least 2 mm depth and (2) a residual myometrium of at least 2.2 mm.
Without a desire to conceive within 1 year.
Signed informed consent.
Exclusion criteria are as follows:
A contraindication for a hysteroscopic niche resection or LNG-IUS 52 mg or not willing to receive this type of treatment.
Women with a positive pregnancy test.
Presence of an intrauterine device.
A contraindication for general or local anaesthesia.
Coagulation disorders that lead to higher risks on bleeding or anticoagulant use.
A (suspected) malignancy, endometrial polyps, atypical endometrial cells, cervical dysplasia, hydrosalpinx that may communicate with the uterus.
Adenomyosis or leiomyoma (the International Federation of Gynecology and Obstetrics (FIGO) leiomyomia subclassification system Type 0, 1, 2, 3) or large leiomyomas causing the uterine cavity of ≥9 cm that examined by transvaginal ultrasound or MRI.
Endocrine disorders that interfere with the menstrual cycle.
Irregular menstrual cycle (>35 days or intercycle variation of 2 weeks or more).
Recruitment and randomisation
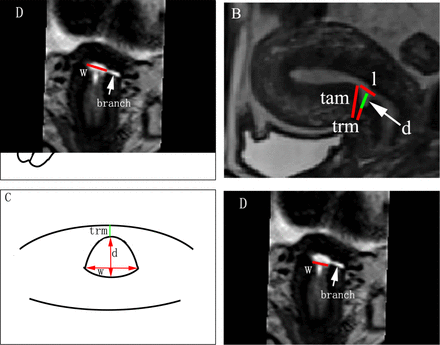
Three-dimensional (3D) MRI will be performed as part of the routine diagnostic workup for the identification of niche (figure 1).
Niche measurement in the 3D MRI. (A, B) Sagittal plane; (C, D) transversal plane. Line l: the length of niche, measured from the upper rim to the lower rim of niche; line d: the depth of niche, measured from the usual limit of uterine limit to the apex of niche; line w: the width of niche, measured the largest width of niche in the transverse plane; line TRM: the thickness of residual myometrium, measured from the apex of niche until the serosa; line TAM: the thickness of adjacent myometrium, measured from the upper endpoint of niche to the serosa; branch: the lateral branch was defined as the branch of niche, of which the widest part <2 mm. TAM, thickness of adjacent myometrium; TRM, thickness of residual myometrium.
Potential eligible patients visiting the outpatient department because of niche-related postmenstrual spotting and in whom transvaginal ultrasound has shown a niche of at least 2 mm depth will be asked to participate in the trial. After the patients complete the informed consent, the examinations and tests will be performed in an outpatient setting by the hospital protocol. The results of these tests will be checked by the principal investigators. After confirming the presence of the niche and matching the inclusion criteria, women will be randomised for LNG-IUS 52 mg or hysteroscopic resection. Randomisation will be performed by using a web-based Research Electronic Data Capture (REDCap) system. From an ethical perspective, we decided to design the Mirena (LNG-IUS 52mg)-Hysteroscopic niche resection niche trial (MIHYS NICHE trial) as an open-label trial both for patients and researchers, but we used a blind method to evaluate the effect of treatment.
Niche evaluation
A 1.5 Tesla (Siemens Avanto, Erlangen, Germany) MRI system will be used. Both T1-weighted and T2-weighted images will be used. Imaging measurement will be performed by two experienced observers who are blinded to the clinical data. Niche will be measured both in the sagittal and in the transversal plane, according to a standardised protocol as recommended by the international niche task force group.24 The length, width and depth of niche, the thickness of residual myometrium (TRM), the thickness of adjacent myometrium, the presence of any branches, the polyp-like structures, the cyst-like formations in the niche will be registered.
Intervention
Intervention of the LNG-IUS 52 mg group
The LNG-IUS 52 mg will be inserted in an outpatient setting by an experienced gynaecologist. After measuring the length of the uterine cavity and the dilatation of the cervical canal, an endometrial biopsy will be taken for histological examination. According to the instructions of LNG-IUS 52 mg and Chinese experts’ consensus for clinical application of LNG-IUS 52 mg, LNG-IUS 52 mg will be inserted with the provided inserter into the uterine cavity within 7 days after the onset of the menstruation under ultrasound monitoring carefully. If needed, a paracervical block will be placed. Ultrasound monitoring will be used to ensure the correct localisation of the LNG-IUS 52 mg. According to the protocol, patients will not receive any additional hysteroscopic resection during a period of at least 6 months.
Intervention of the hysteroscopic niche resection group
Patients allocated for hysteroscopic niche resection will undergo the procedure under general anaesthesia in the day care setting. After dilatation of the endocervix up to Hegar 9 mm, the hysteroscopic resection will be performed using a 9 mm resectoscope in a standardised way, as described previously.6 For uterine distention, 0.9% NaCl at a pressure of 100–120 mm Hg will be used. The niche will be evaluated by hysteroscopy according to a standardised way (presence of dome-shaped scar defect, of nodule-like endometrial hyperplasia, and of vascularity, valve-like motion or a very high edge). After hysteroscopic evaluation, the endometrium specimens will be collected for histological examination. A cutting loop will be used to resect the distal rim of niche6 and the niche surface including all small vessels will be coagulated.
Hysteroscopic resection will be performed by two gynaecologists with extensive experience in hysteroscopic niche resections. The two gynaecologists have completed the learning curve (JZ and YL).
Patient and public involvement
Neither the patients nor the public will be involved in the study design. They will also not be involved in the recruitment process or the conduct of the study. The results will be disseminated to patients via an open access publication and our local trials teams.
Outcome measures
Primary outcome measures
The primary outcome is the effectiveness in reducing postmenstrual spotting during the menstrual cycle 6 months after randomisation. Postmenstrual spotting is defined as 2 or more days of intermenstrual spotting or as 2 or more days of brownish discharge immediately after the menstrual period or irregular light flow bleeding in case the total period of the menstrual bleeding exceeds 9 days. If the bleeding period is shorter than 10 days, the brownish discharge is not calculated as spotting. Effective case is defined as patients with a reduction of spotting by 50% or more. Effective rate is defined as the number of effective cases in the total number of follow-up cases. In the LNG-IUS 52 mg group, if there is a regular menstrual cycle, the definition as reported above is used. If the bleeding pattern is irregular, the total unpredictable bleeding or spotting days per months (outside normal period) will be taken.
Secondary outcome measures
Menstrual pattern (menstrual cycle, duration of the menstruation), postmenstrual spotting, intermenstrual spotting, irregular light flow bleeding, total bleeding days per month,6 rate of amenorrhoea(amenorrhoea was defined as complete absence of bleeding or spotting for at least 3 months).25
Total spotting days during one menstrual cycle 12 months after randomisation.
Visual Analogue Scale (VAS) for discomfort from spotting and chronic pelvic pain, urological symptoms.26
Women’s satisfaction with treatment (five-point Likert scale, very unsatisfied, unsatisfied, neutral, satisfied, very satisfied).27
Hormonal-related side effects such as mood changes, weight gain, breast pain and libido.28
Direct economic costs (including the costs of preoperative examination, medical consumption and the surgery).
Complications (including urinary tract injury, fever, infection) and applied cointerventions.
Menstrual characteristics were self-recorded in a menstruation diary at 3, 6, 9 and 12 months after inclusion and uploaded in an electronic data system.
Statistical consideration
Sample size calculation
Based on our previous prospective cohort study executed in 30:30 patients, the effective rate at 6 months after treatment of LNG-IUS 52 mg was 87.5%, while that of hysteroscopic niche resection was 73.3%. To show the superiority of LNG-IUS 52 mg group as compared with hysteroscopic niche resection group, with 1:1 allocation ratio, 80% statistical power and a two-sided significance level of 5%, a total of 188 patients (94 in each group) will need to be enrolled . Thus, 208 women (104 in each group) will need to be randomised,including 10% lost to follow-up or protocol variation.
Statistical methods
Analyses will be performed according to the intention-to-treat principle and additionally by per-protocol analysis. For continues variable, if normally distributed with equal variances we will use Student’s t-tests; otherwise Mann-Whitney U tests will be used. For categorical variables, we will use χ2 tests or Fisher’s exact test as appropriate. For categorical outcome, we will conduct a logistic regression analysis, in which we compare the factors of the effective group versus the control group. All tests will be performed two sided and a p<0.05 will be considered as statistically significant.
Data collection and management
At baseline obstetric history, indication for the CS(s), gestational age at time of CS(s), gynaecological symptoms before and after the last CS, menstrual pattern, previous therapies including medication or previous uterine surgery will be registered. The patient will receive digital secured questionnaires at baseline, 3, 6, 9 and 12 months after randomisation about their menstrual pattern. The questionnaires include a validated menstrual bleeding score chart,6 VAS for discomfort from spotting, the severity of chronic pelvic pain,26 satisfaction with treatment (five-point Likert scale),27 and side effects of LNG-IUS 52 mg. Medical consultation, medication use and received additional treatments will be reported in an online diary. Any received additional treatment will be recorded in the case report file.
If applicable, surgical outcomes such as satisfaction of the gynaecologist, surgical steps, complications and postoperative recovery will be recorded in the case report file. All additional therapies or transfer to another therapy after the first intervention will be registered in both groups, such as oral contraceptives or second surgical treatment.
Follow-up will be performed in both groups 3, 6, 9 and 12 months after randomisation, all patients will undergo transvaginal sonography to record all niche features at baseline and/or LNG-IUS 52 mg position (figure 2).
Flow chart. *Questionnaires: menstrual pattern, validated menstrual bleeding score chart,6 VAS score for discomfort from spotting, chronic pelvic pain,26 discomfort due to spotting and satisfaction with treatment (five-point Likert scale),27 side effects or complications after therapy. LNG-IUS, levonorgestrel intrauterine system; VAS, Visual Analogue Scale.
Interim analysis and safety monitoring
Because of the relatively small sample size and the expected duration of inclusion, an interim analysis is not planned.
Hysteroscopy and LNG-IUS 52 mg insertion are both common practised procedures in gynaecology. Although both procedures are routine, complications may occur after the operation. The complications can be divided into two categories: complications associated with procedures and complications associated with distension media. Complications associated with procedures include uterine and bladder perforation, cervical laceration, pelvic infection and haemorrhage.29 Complications associated with distension media can be prevented by monitoring the usage and fluid loss during the surgery.30
All serious adverse events will be reported to the Medical Ethics Committee. The Medical Ethics Committee can terminate the trial duly for safety reasons. Every observed or reported adverse event that link to suspected causal relationship with surgery or treatment will be registered. All adverse events will be followed until they have abated, or until a stable situation has been reached. Depending on the event, the follow-up may require additional tests or medical procedures as indicated.
Discussion
Strengths and limitations
The MIHYS NICHE trial is the first randomised controlled trial that will provide evidence for the effectiveness of LNG-IUS 52 mg versus hysteroscopic niche resection in reducing niche-related spotting symptoms.The trial is adequately powered based on a representative cohort study. Randomisation is performed with a use of allocation concealment with the help of web-based REDCap system, which will reduce the chance for bias.
We design MIHYS NICHE trial as an open-label trial both for patients and for researchers, but we used a blind method to evaluate the effect of treatment. This can reduce the bias of evaluation and ensure the authenticity of evaluation effect.
The MRI of CSD has not been commonly reported because of the price and little demand for investigations of AUB or other noncancerous pathologies.31 However, MRI has the advantages of high sensitivity, high resolution between different soft tissues and arbitrary section imaging, while the 3D MRI with the section of only 1 mm and 3D reconstruction are more accurate for the evaluation of lesions with fine anatomical structure, minor lesions such as branches which can be missed by traditional MRI.
In most previous studies, hysteroscopic niche treatment is performed in patients with residual myometrium of at least 2.5–3 mm to avoid uterine perforation and bladder injury.6 28–35 However, some studies used 2 mm as the cut-off standard of residual myometrium in the hysteroscopic treatment.22 35 Based on our previous large cohort study, a minimal TRM of 2.2 mm resulted in significantly better outcomes after hysteroscopic niche resection.36 For this reason, we excluded women with a TRM of <2.2 mm. A strength of the study is that the learning curve was completed before starting this study. Two experienced gynaecologists performed more than 400 hysteroscopic niche resections together, so far related complications have not been reported.
Potential impact and implications
Although niche-related symptoms have been acknowledged and various treatments have been implemented in recent years, there is still a lack of studies of evaluating the effect of those treatments. LNG-IUS 52 mg has advantages that it does not require (general) anaesthesia or hospitalisation and that it is easy to insert. LNG-IUS 52 mg also has the superiority of low risks of complications and low costs. However, it has not been supported by prospective studies so far whether LNG-IUS 52 mg is superior over a hysteroscopic niche resection.11 12 14–17 22 Therefore, this randomised controlled study will provide the highest level of evidence-based medicine evidence for the treatment niche-related symptoms.
Ethics and dissemination
The study was approved by the local medical ethics committee and by the Institutional Review Board of the International Peace Maternity and Child Health Hospital, Shanghai, China (No. GKLW 2019-08). Participants will sign a written informed consent before participation. The results of this study will provide evidence for LNG-IUS 52 mg in treating women with niche-related postmenstrual spotting.The results of this study will be submitted to a peer-reviewed journal for publication.
Ethics statements
References
Footnotes
CH and XH are joint first authors.
Contributors JZ, JH and BWM designed the study. LX performed the sample size contributed to supervise the study and statistical analysis. TS critically revised the manuscript and contributed to the 3D MRI imaging. CH and XH wrote the manuscript. YL contributed to follow up the patients; CZ, LY and XZ contributed to data collection. All authors critically revised this final version for publication. All authors read and approved the final version of the manuscript.
Funding This work was supported by National Key Research and Development Programme (2018YFC1002102), Research Project of Shanghai Health and Fitness Commission (201940012,20184Y0344)),Shanghai Municipal Key Clinical Specialty (shslczdzk01802), Medical Engineering Cross Funds from Shanghai Jiao Tong University (YG2017QN38, ZH2018QNA36, YG2021ZD31), Medical innovation research project of the 2020 "Science and Technology Innovation Action Plan" of Shanghai Science and Technology Commission (20Y11907700), and Clinical Science and Technology Innovation Project of Shanghai Hospital Development Center(SHDC22020216).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.