Article Text
Abstract
Patients with inflammatory conditions are at high risk for cardiovascular (CV) disease. Despite such elevated risk, their CV risk factors are suboptimally managed.
Objective To evaluate the effect of a pharmacist-led intervention on CV risk in patients with inflammatory conditions.
Design Prospective pre–postintervention.
Setting 17 community pharmacies across Alberta.
Population Adults with inflammatory conditions (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, gout, systemic lupus erythematosus, psoriasis vulgaris) who had at least one uncontrolled risk factor (A1C, blood pressure, LDL-cholesterol or current tobacco users).
Intervention All patients enrolled in the study received: physical and laboratory assessment, individualised CV risk assessment and education regarding this risk, treatment recommendations, prescription adaptation and prescribing where necessary to meet treatment targets, regular communication with the patient’s treating physician(s) and regular follow-up with all patients every month for 6 months.
Outcomes Primary: change in estimated CV risk (risk of a major CV event in the next 10 years) after 6 months. Secondary: change in individual risk factors (blood pressure, LDL-cholesterol, A1C and tobacco cessation) over a 6-month period.
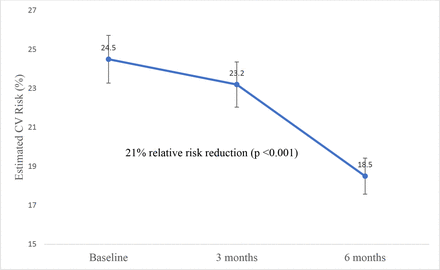
Results We enrolled 99 patients. The median age was 66.41 years (IQR 57.64–72.79), More than half of them (61%) were female and more than three-quarters (86%) were Caucasians. After adjusting for age, sex and ethnicity and centre effect, there was a reduction of 24.5% in CV risk (p<0.001); including a reduction of 0.3 mmol/L in LDL-c (p<0.001), 10.7 mm Hg in systolic blood pressure (p<0.001), 1.25% in A1C (p<0.001). There was a non-significant trend towards tobacco cessation.
Conclusion This is the first study on CV risk reduction in patients with inflammatory conditions in a community pharmacy setting. RxIALTA provides evidence for the benefit of pharmacist care on global cardiovascular risk reduction as well as the individual cardiovascular risk factors in patients with inflammatory conditions.
Trial registration number NCT03152396.
- rheumatology
- public health
- general diabetes
- hypertension
- cardiac epidemiology
- lipid disorders
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first study to assess the effect of a pharmacist-led case finding and care on cardiovascular (CV) risk in patients with chronic inflammatory conditions in a community pharmacy setting.
The pharmacist-led case finding and care enhanced access to CV risk assessment and care in a high-risk population that otherwise would not have their CV risk assessed.
The pharmacist-led case finding and care (including prescribing and ordering laboratory tests) was associated with CV risk reduction and improvement in all the individual CV disease risk factors.
Introduction
Cardiovascular disease (CVD) is one of the leading causes of morbidity and mortality worldwide and in Canada accounting for nearly one-third of the total deaths.1 2 The majority of CVD cases are caused by modifiable risk factors such as tobacco use, obesity, hypertension, hyperlipidaemia, diabetes and physical inactivity.3 Chronic inflammatory diseases, such as rheumatoid arthritis, psoriatic arthritis ankylosing spondylitis, gout, systemic lupus erythematosus and psoriasis, are also increasingly being recognised as independent risk factors for CVD.4–7 Indeed, it has been reported that the risk of myocardial infarction, heart failure and CV death among patients with chronic inflammatory disease is twofold to threefold greater than in the general population.8–10 Such increased risk can be explained by the combined impact of systemic inflammation, burden of traditional CVD risk factors and impact of certain medications (eg, steroids, non-steroidal anti-inflammatories (NSAIDs), retinoids).5 6
Despite being recommended by international guidelines,7 CV risk assessment has not been incorporated into many clinicians’ daily routine.7 In fact, reports indicate that such assessments generally only exist in larger centres for non-rheumatology patients.11–13 Moreover, Keeling et al reported that most rheumatologists, who are the main caregivers for patients with these conditions, conducted suboptimal CV risk assessments.14 Unfortunately, this gap in care is not consistently absorbed by family physicians due to lack of recognition of CV risk in these patients and competing demands of other healthcare needs.7 Furthermore, many patients, especially those who are living in remote or rural areas, do not have access to family physicians.15 These facts, combined with the benefits of early identification after the diagnosis,16 highlight the need for new and innovative ways for assessing CV risk in this high-risk population.
Special considerations need to be taken into account when calculating CV risk in patients with chronic inflammatory diseases, as the ‘classic’ risk engines (such as Framingham17 might underestimate the overall risk,18 since they have not been adequately evaluated in this patient population.5 19 For example, those patients who might benefit from lipid-lowering agents may be categorised ‘low risk’ when using the Framingham risk engine.18 As such, it has been recommended to use a modified Framingham risk engine (multiply the overall risk by 1.5) in this patient population.20 There is conflicting evidence in the literature regarding lipid panel measurements in patients with rheumatoid arthritis. Some studies reported that total cholesterol and LDL-cholesterol are significantly lower, while other studies reported that they are significantly higher in patients with rheumatoid arthritis when compared with the general population.21–23 Despite the variation, it is still recommended to treat patients with rheumatoid arthritis to general population lipid targets with consideration of risk modification, such as the European League Against Rheumatism recommendations that suggest multiplying the CV risk score by a factor of 1.5 in these patients.24 25
Pharmacists are front line, accessible, primary healthcare professionals who see patients at risk/with chronic conditions more frequently than any other healthcare provider.26 The efficacy of their interventions in chronic diseases including diabetes,27 dyslipidaemia,28 hypertension,29–32 heart failure33 and CVD34–36 has been well demonstrated in the literature. Pharmacists can systematically identify patients at high risk of CVD,36 help manage their condition, improve their medication use31 32 37 and assist them to achieve their treatment targets.27–32 In addition to clinical outcomes, pharmacist interventions are also associated with high levels of patient satisfaction, improved adherence to therapy and considerable cost savings and efficient use of healthcare resources.31 32 38–40 This evidence, coupled with their full scope of practice including prescribing and laboratory test monitoring, ideally position pharmacists to conduct CV risk assessment and management. Therefore, we conducted this study to determine the effect of a pharmacist-led intervention on CV risk in patients with chronic inflammatory diseases.
Methods
RxIALTA was a non-randomised prospective pre–postintervention study that was conducted in 17 community pharmacies across Alberta, Canada (for a list of the participating pharmacies please see the acknowledgement section). We sed a non-randomised design because our previous work in pharmacist-led CV risk reduction,36 a 723 patient (those with diabetes, chronic kidney disease, established vascular disease or Framingham risk >20%) randomised trial demonstrated significant reductions in estimated cardiovascular risk, and it was felt unethical to randomise this underserved high-risk population to usual care.
Patients were included if they were adults (≥18 years of age) with a physician-diagnosed chronic inflammatory condition (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, gout, systemic lupus erythematosus or psoriasis) and had at least one uncontrolled risk factor (blood pressure (≥140/90 without diabetes;≥130/80 with diabetes),41 LDL-cholesterol (>2.0 mmol/L),42 A1C (>7.0%)43 or current tobacco use). We excluded patients if they were unwilling to participate/sign the consent form, unwilling or unable to participate in regular follow-up visits, pregnant or experiencing a disease exacerbation (this may be indicated by current treatment with high or tapering dose of steroids), since lipid panel is most accurately measured when inflammatory diseases are stable or in remission.5
Recruitment
Pharmacists and pharmacy staff used the following methods to identify potential patients: (1) Proactive case finding: patients with physician-diagnosed chronic inflammatory conditions were identified by reviewing prescriptions of diseasemodifying antirheumatic drugs, NSAIDs, immunosuppressants, gout medications, biologics (eg, adalimumab, infliximab, ustekinumab, ixekizumab, secukinumab) and/or topical drugs containing calcipotriol, methotrexate with a rheumatologist or a dermatologist prescriber; (2) Case finding via in-pharmacy posters and weekly fliers and (3) Case finding via bag stuffers with the above medications.
As part of routine care, pharmacists measured the blood pressure and checked the most recent laboratory test results for the identified patients (through the provincial electronic health record). They then checked whether patients met the inclusion criteria. The pharmacists explained the study to those who met the inclusion criteria and invited them to take part. Patients who agreed to take part were asked to sign a written informed consent form. Once the signed written informed consent form was obtained the patients were enrolled in the study.
The patient’s physician(s) received a letter from the pharmacist to inform them that the patient agreed to participate in this study.
Intervention
All enrolled patients received: (1) Patient assessment (blood pressure measurement according to Hypertension Canada guidelines,41 waist circumference, weight and height measurements), (2) Laboratory assessment of A1C, non-fasting lipid panel (total cholesterol, LDL-cholesterol and HDL-cholesterol) and kidney function and status (creatinine (and estimated glomerular filtration rate), random urine albumin to creatinine ratio), (3) Individualised CV risk assessment and education regarding this risk using a validated interactive online tool36 that explains the individual’s CV risk, the contribution of each risk factor to the overall risk and the impact of the intervention and controlling the risk factors on the overall CV risk (https://www.epicore.ualberta.ca/epirxisk/), (4) Treatment recommendations, prescription adaptation and prescribing where necessary to meet guideline recommended targets. Pharmacists practised to their full scope (including prescribing medications and ordering and interpreting laboratory tests when needed), (5) Regular monthly follow-up for 6 months to check on patients’ progress and provide ongoing care and motivation; and (6) Regular communication with the patient’s physician(s) after each contact with the patient as per usual pharmacist practice.
Patient and public involvement
No patient involved
Outcomes
The primary outcome was the change in CV risk over a 6-month period. CV risk is defined as the risk for future CV events (coronary heart disease, stroke, peripheral arterial disease)7 8 as calculated by validated risk assessment equations. The CV risk was calculated using EPI·RxISK Cardiovascular Risk Calculator (https://www.epicore.ualberta.ca/epirxisk/). It was estimated using the Modified Framingham20 risk assessment equation (Framingham risk score multiplied by 1.5) for patients who have chronic inflammatory conditions without other comorbidities. If the patient had other CV risk-modifying conditions (diabetes, previous vascular disease or chronic kidney disease), risk was calculated using the Modified Framingham20 and the most appropriate risk assessment equation based on the patient’s medical history. The UK Prospective Diabetes Study44 risk assessment equation was used for those with diabetes, SMART risk assessment equation45 was used for patients with previous vascular disease and Framingham17 risk assessment equation was used for the ones with chronic kidney disease. If the patient had both chronic inflammatory conditions and other CV risk-modifying conditions, the risk was calculated using all the respective risk assessment equations, and the risk assessment equation estimating the highest risk was used.
The secondary outcomes were the change in individual risk factors (blood pressure (in patients with hypertension), LDL-cholesterol (in patients with dyslipidaemia), A1C (in patients with diabetes) and tobacco cessation (self-reported abstinence)) over a 6-month period.
Sample size and analytical plan
Sample size
Using the information from our previous pharmacist-led CV risk reduction trial, RxEACH36 (Baseline CV risk (26.2%) and standard deviation (SD) (17.8)) and the following assumptions of 80% power and alpha of 0.05, 89 patients were required to detect 21% risk reduction. The sample size was inflated to 100 to to account for possible dropouts, lost to follow-up and withdrawals of consent.
Analytical plan
Analysis was performed by using R V.3.6.2 (Vienna, Austria; https://www.R-project.org/) and SAS V.9.4 software (SAS Institute).
Data were first screened to confirm that all the participating patients met the inclusion/exclusion criteria and provided informed consent. Once those conditions were confirmed, statistical analysis started.
Demographic information and clinical characteristics were analysed using descriptive statistics. Frequency (percentage) was used for categorical variables and mean (SD) for continuous variables. Statistical significance at the univariable level was assessed using χ2 test or Fisher’s exact test (when small frequencies present) for categorical variables, and t-test for continuous variables (assumption of statistics tests were checked ahead). The primary outcome was analysed by paired t-test. Multivariable linear mixed effect models were used to adjust for centre effect, age, sex and ethnicity. Secondary outcomes were analysed using paired t-test and χ2 test as appropriate.
Trial and data management was performed by EPICORE Centre.
Results
The study was launched in August 2017, and the last patient was enrolled in July 2019. Follow-up was completed in January 2020. We screened 126 patients, of those 103 were eligible. We enrolled 99 patients and 94 of them completed the study (figure 1). Demographic and clinical characteristics are presented in table 1. Mean age was 64 years (SD 14.8), approximately two-thirds (61%) of the participants were female and 86% were Caucasian. More than half (56%) had rheumatoid arthritis, 14% had psoriasis, 12% had psoriatic arthritis, 11% had gout, 6% had ankylosing spondylitis and 1% had systemic lupus erythematosus. Hypertension was the most commonly reported risk factor (47%), followed by dyslipidaemia (45%), diabetes (13%), atherosclerotic vascular events (angina, heart attack, stroke/TIA) (12%), current tobacco use (11%) and chronic kidney disease (9%). In addition, average body mass index was 28.2 (5.2) kg/m2 and only 9% reported exercising for 30 min (or more) five or more times per week. Importantly, only 2% of participants reported that their CV risk was assessed by a healthcare provider before taking part in the study.
Baseline demographic and clinical characteristics
Study flow chart.
Estimated CV risk was reduced from 25% (SD 16.1) at baseline to 19.8% (SD 14.7) after 6 months. After adjusting for age, sex, ethnicity and centre effect, such reduction corresponded to a 24.5% relative risk reduction (6 (95% CI (4.6 to 7.4)) p<0.001) (figure 2). In patients with hypertension, significant reductions were observed in systolic and diastolic blood pressure (table 2). Similarly, we noted reductions in LDL-cholesterol in patients with dyslipidaemia and A1C in those with diabetes (table 2). Participants’ dietary habits were also improved (p=0.02), while exercise, alcohol and tobacco use were not significantly changed.
Changes in individual risk factors
Change in estimated CV risk over time. CV, cardiovascular.
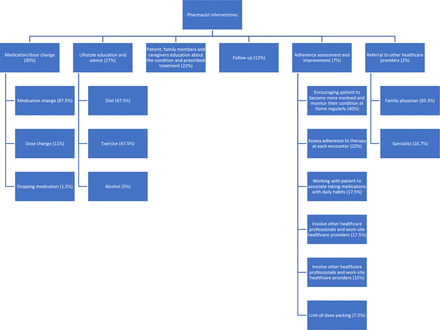
Pharmacist interventions are listed in figure 3. Medication/dose change was the most implemented intervention (30%), followed by lifestyle education and advice (27%), patient, family members and caregivers’ education about the condition and prescribed treatment (22%), follow-up (12%), adherence assessment and improvement (7%) and referral to other healthcare providers (2%). There were very minimal adverse events reported during the study.
Pharmacist interventions.
Discussion
Chronic inflammatory conditions increase patient’s risk for CV events; however, these patients are often not receiving CV risk assessment or treatment. We hypothesised that community pharmacists could proactively and systematically screen for chronic inflammatory diseases (because of the unique medications used in these conditions), and then manage their CV risk factors. We found that a pharmacist-led care reduced the risk of major CV events by 24.5% (p<0.001) over a 6-month period. The intervention was also associated with reductions in blood pressure, LDL-cholesterol and A1C. Such improvements are related to the following pharmacist activities: medication/dose changes, lifestyle education and advice, patient, family members and caregivers’ education about the condition and prescribed treatment, follow-up, adherence assessment and improvement and referral to other healthcare providers.
Our findings are consistent with the findings of the RxEACH study, which evaluated the impact of pharmacist intervention (assessment, prescribing and follow-up) on CV risk in patients at high risk for CVD (patients with diabetes, chronic kidney disease, established vascular disease or Framingham risk >20%). RxEACH reported that such intervention was associated with CV risk reduction as well as improvements in all individual risk factors.36
Our findings are also consistent with the findings of Semb et al who reported significant CV risk reduction when a CV risk factor (lipids) was managed appropriately.21 They also highlight the importance of pharmacist prescribing, as ‘medication/dose change’ was the most implemented intervention. This intervention would have not been possible without having independent prescriptive authority. These findings are supported by the findings of Al Hamarneh et al and Wubben and Vivian who reported that better outcomes were achieved when pharmacists had prescriptive authority.46 47
This study is not without limitations. As described above, the study was not a randomised controlled trial, due to ethical concerns of randomising this high-risk underserved population to usual care after proving that the intervention is effective. We acknowledge that this reduces causal inference, however, the findings of this study are similar to the randomised RxEACH study.36 Since the 6-month follow-up period can be considered relatively short; it is possible that the effects of the intervention could be short lived. It is also possible, however, that greater improvements leading to larger CV risk reduction could have been observed with a longer follow-up period. Pharmacists who provided the intervention also conducted the assessment and entered the information into the study online system where CV risk was calculated. This could have introduced bias; however, the study team monitored study sites against source documents to ensure accuracy. The fact that adverse events were self-reported could have led to under-reporting.
Our findings, combined with the fact that the risk of myocardial infarction, heart failure and CV death among patients with chronic inflammatory diseases is much higher than the general population,8–10 highlight the importance of focusing on the patient as a whole, rather than only focusing on their acute complaints.
It is noteworthy that only 2% of our participants had their CV risk assessed before taking part in the study. This is consistent with the literature, as it has been reported that the levels of awareness and perceived risk of CVD is low in this patient population.48 Gaps in care have also been reported when it comes to CV risk assessment.7 12–14 This also highlights the importance of a systematic and proactive approach towards case finding by pharmacists—as many patients would not know to ask for CV risk assessment. This is a unique feature of involving community pharmacists—an approach which we have used successfully in a number of areas.28 36 49
RxIALTA findings add to the high-level evidence of effective pharmacist prescribing interventions in improving CV risk and individual CVD risk factors.36 49 Such high-level evidence should encourage policy makers to broaden the scope of practice for pharmacists and pharmacy professional organisations to implement those interventions on a larger scale to seize the opportunity to enhance patient care.
To our knowledge, this is the first study to assess the effect of a pharmacist-led case finding and care on CV risk in patients with chronic inflammatory conditions in a community pharmacy setting. We have demonstrated that pharmacist-led intervention (including prescribing) improved CV risk as well as the individual CVD risk factors. Pharmacists also improved the access to care in a high-risk population that otherwise would not have their CV risk assessed. Implementing this on a wider scale could help addressing one of the world’s major public health challenges.
Acknowledgments
None of this could have taken place without the dedication and caring of the RxIALTA investigators, listed in descending order of recruitment:
Nader Hammoud (Shoppers Drug Mart #2326, Calgary), Nataliya Posudwvska (Calgary Co-op, Calgary), Rick Siemens (London Drugs #38, Lethbridge), Dixie Richardson (Safeway Pharmacy, Edmonton), Aileen Coutts (Calgary Co-Op, Calgary), Jan Messiha (Calgary Co-op, Calgary), Farzana Sharif (Calgary Co-Op, Calgary), Pegah Manzoori (Calgary Co-Op, Calgary), Maria James (Calgary Co-Op, Calgary), Jack Dhaliwal (Calgary Co-Op, Calgary), Derek Durocher (Shoppers Drug Mart #313, Edmonton), Leanna St. Onge, Otti Gohrbandt and Chelsey Collinge (Co-op Pharmacy, Rocky Mountain House), Aila Omar (Co-op Pharmacy, Edmonton), Diane Lazarko-Gamache (Calgary Co-Op, Calgary), Sonal Ijner (Calgary Co-Op, Calgary), Carlene Olyksen, Jelena Okuka (Meridian Pharmacy, Stony Plain), Murtaza Hassanali (Shoppers Drug Mart #371, Edmonton), Penny and Fausta (Penny and Fausta Pharmacy, Calgary).
References
Footnotes
Contributors Substantial contribution to study conception and design: YNAH, RG, SK, CM, AM and RT. Substantial contribution to data collection: YNAA, AM and RT. Substantial contribution to data analysis and interpretation: YNAH and RT. Drafting the article or revising it critically: YNAH, RG, SK, CM, AM and RT. Final approval of the submitted version: YNAH, RG, SK, CM, AM and RT.
Funding We would like to acknowledge the generous support of the funder of RxIALTA: Canadian Initiative for Outcomes in Rheumatology cAre (CIORA) (grant number: 2015–001).
Disclaimer Our funder did not have any role in the study design, collection, analysis, interpretation of the data, writing the report and the decision to submit for publication. We would like to acknowledge the support of the Consultation and Research Services Platform at The Alberta' SPOR SUPPORT Unit in Data management and statistical services (Grant number N/A).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval RxIALTA was approved by the Health Research Ethics Board of the University of Alberta (Pro00072858).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request. Data will be available on reasonable request.