Article Text
Abstract
Objectives To investigate the associations between echocardiographic left atrial (LA) size and incident stoke and stroke cause mortality among a rural population in China.
Design A prospective study.
Setting and participants Based on the Northeast China Rural Cardiovascular Health Study, we selected a total of 10 041 participants aged ≥35 years who agreed to have transthoracic echocardiography at baseline and were successfully followed up for incident stoke and stroke cause mortality.
Primary outcome measure The outcomes were stroke and stroke cause death according to medical records and death certificates during the follow-up period.
Results LA enlargement (LAE) group had a higher prevalence of cardiovascular disease than normal LA diameter (LAD) group. After excluding individuals who had a prior stroke, subjects with LAE showed higher incident rates of stroke and its mortality in the overall and specific stratified analyses (all p<0.05). Kaplan-Meier analysis revealed that LAE could predict stroke incidence and stroke-free survival, but the association was no longer observed after the adjustment for potential confounding factors. Cox regression analysis reported that per 1 SD increment in LAD and LAD/body surface area (BSA) was associated with an increased incidence of stroke (LAD: HR=1.20, 95% CI 1.08 to 1.33, p<0.001; LAD/BSA: HR=1.22, 95% CI 1.11 to 1.35, p<0.001) and stroke cause mortality (LAD: HR=1.27, 95% CI 1.08 to 1.50, p<0.01; LAD/BSA: HR=1.41, 95% CI 1.20 to 1.65, p<0.001) in the total population, and similar trends were found in both genders (all p<0.05). LAD or LAD/BSA was related to ischaemic and haemorrhagic stroke incidence, and the risk of ischaemic and haemorrhagic stroke mortality (all p<0.05). The dose–response curves further suggested linear associations between LAD, LAD/BSA and the incidence of stroke and subsequent mortality in the general population (all p<0.05).
Conclusions Our population-based study implied that LA size, especially LAD and LAD/BSA, might be useful echocardiographic biomarkers that had the potential to predict incident stroke and stroke cause mortality.
- epidemiology
- echocardiography
- stroke medicine
- cardiac epidemiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This was a large population-based prospective study, providing adequate data and sample size to delineate the study objective.
The left atrial (LA) size determined by transthoracic echocardiography was treated as dichotomous and continuous variables, respectively.
Multiple clinical covariates and echocardiographic parameters were adjusted in Cox proportional hazards models and multivariate logistic regression analyses.
Restricted cubic spline functions were applied to draw the dose–response curves that indicated the associations between LA diameter (LAD), LAD/body surface area (BSA) and the risk of stroke or stroke cause mortality.
This was a single centre study and further multi-centre prospective ones with longer periods of follow-up should be conducted.
Introduction
Stroke is a global catastrophic condition with a great burden of disability and mortality. It has been predicted that there will be almost 12 million stroke deaths and 70 million stroke survivors by 2030.1 2 Pathologically, stroke can be classified into ischaemic stroke (around 80%) and haemorrhage stroke (around 20%).3 To discern the high risks of stroke subjects and well manage stroke risk factors has a crucial individual and social impact.4
Left atrial (LA) dilation, the hallmark of LA remodelling, has been noted to be of clinical value for predicting the likelihood of cardiovascular events and all-cause mortality beyond potential risk factors.5 6 Among various quantification methods to assess LA size, the antero-posterior LA diameter (LAD) determined by transthoracic echocardiography is known to be the simplest and most reproducible measurement, and widely used in daily clinical practice and research.7 As a risk factor for ischaemic stroke, LA enlargement (LAE) has been discussed in many studies, but the data from large-scale prospective cohorts are limited and conclusions vary. Several longitudinal studies identified that LAD was associated with incident stroke,4 8 9 but other cohort studies found no relationship.10–13 Some authors also investigated whether this association was influenced by gender. Benjamin et al and Di Tullio et al revealed an independently predictive effect of LAE on ischaemic stroke risk only in men,14 15 whereas others found that LAE predicted stroke only in women.11 16 However, above researches just concentrated on non-Asian populations, and there were a lack of data regarding the importance of LA size in predicting incident stoke in Asians. Additionally, a link of LA size to stroke cause mortality and haemorrhagic stroke incidence has not been sufficiently described in the literature. Up to now, only one Asian community cohort from Japan demonstrated that LAE was an independent predictor of stroke/systemic embolism in patients with atrial fibrillation (AF).17 Considering the differences in epidemiological characteristics, the mechanisms of strokes in China can also vary from other countries. Therefore, it is the necessity to discern the association between LA size and incident stroke and its mortality in a Chinese population.
In the current study, based on the Northeast China Rural Cardiovascular Health Study (NCRCHS) with a median followed up of 4.66 years, we aimed to determine the role of LA size, regarded as dichotomous and continuous variables respectively, on the prediction of incident stroke and stroke cause mortality in the total and stratified analyses. Then, we sought to find out whether there were some continuous dose–response associations between LAD, LAD/body surface area (BSA) and the risk of stroke and its mortality in the general population. Our data may serve to enhance the risk stratification for stroke, and help guide targeting of primary prevention efforts.
Materials and methods
Study population
NCRCHS is a community-based prospective cohort study carried out in rural areas of Northeast China. The design and inclusion criteria of the study have been described previously.18 19 In brief, a total of 11 956 participants aged ≥35 years were recruited from Dawa, Zhangwu and Liaoyang counties in Liaoning province between 2012 and 2013, using a multi-stage, randomly stratified and cluster-sampling scheme. We excluded 341 subjects who refused to have an echocardiography performed at baseline. In 2015 and 2017, participants were invited to attend a follow-up study. Of the 11 615 subjects, 10 377 participants consented and were eligible for our follow-up study. A total of 10 041 participants (96.8%) completed at least one follow-up visit and were available for our analysis. Detailed information was collected at baseline for each participant.
Data collection
At baseline, detailed information on demographic characteristics, lifestyle factors and medical history were acquired by interview with a standardised questionnaire. Smoking was defined as having smoked at least one cigarette per day for more than 6 months. Drinking was defined as having alcohol consumption at least two times a week for more than 1 year. Weight and height were measured with participants in light weight clothing and without shoes. BSA was calculated as (0.0061×height (cm)+0.0128×wt (kg)−0.1529). Blood pressure was assessed three times with participants seated after at least 5 min of rest using a standardised automatic electronic sphygmomanometer (HEM-907; Omron, Tokyo, Japan). Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg, and/or use of antihypertensive medications.20 Fasting blood samples were collected in the morning from participants who had fasted at least 12 hours. Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose (FPG), serum creatinine and other routine blood biochemical indexes were analysed enzymatically. Diabetes was defined as FPG ≥7 mmol/L (126 mg/dL) and/or being on medication for diabetes.21 Dyslipidaemia was defined as serum TC ≥6.21 mmol/L (240 mg/dL), or TG ≥2.26 mmol/L (200 mg/dL), or LDL-C ≥4.16 mmol/L (160 mg/dL), or HDL-C <1.03 mmol/L (40 mg/dL) and/or under taking hypolipidaemic drugs.22
Transthoracic echocardiographic examination was performed using a commercially available Doppler echocardiograph (Vivid, GE Healthcare, USA) with a 3.0 MHz transducer, including M-mode, two-dimensional, spectral and colour Doppler. The echo did not have a clinical indication, but rather was done as part of a longitudinal cohort study at specific study visits. Echocardiographic analyses and readings were conducted by three doctors specialised in echocardiography, and there was a high degree of intra-observer and inter-observer reproducibility for interpretation of the echoes. Under the guideline of the American Society of Echocardiography,7 the parasternal long-axis view was measured to record antero-posterior LAD, interventricular septal thickness, left ventricular (LV) end-diastolic internal dimension (LVIDd), LV end-systolic internal dimension (LVIDs) and posterior wall thickness (PWTd). LV mass (LVM) was calculated by the formula: LVM=0.8×[1.04{(IVSTd+PWTd+LVIDd)3–LVIDd3}]+0.6 g. LVM was divided by BSA to acquire LVM index (LVMI). Normally, the LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were estimated by Teichholz equations: LVEDV (mL)=LVIDd3×7.0/(2.4+LVIDd), LVESV (mL)=LVIDs3×7.0/(2.4+LVIDs). When there were abnormalities in cardiac structure and function, we used the biplane Simpson’s rule for volume calculations from both the apical four-chamber and two-chamber views. LV ejection fraction (LVEF) was calculated as [(LVEDV−LVESV)/LVEDV]×100%. We applied pulsed-wave Doppler to record the early diastolic peak flow (E) of mitral valve in the apical four-chamber view. Pulsed-wave tissue Doppler imaging was used to assess the peak early (e′) diastolic velocities of the septal and lateral mitral annulus in the apical four-chamber view. Subsequently, the average from the septal and lateral velocities was used to obtain the E/e′. LAE was defined as LAD >39 mm and >37 mm for men and women, respectively.23
Judgment and definition of clinical outcomes
The median follow-up time was 4.66 (4.36–4.93) years. The number of effective follow-up cases was 10041, and follow-up rate was 96.8%. In the present study, an incident event was defined as a new onset of stroke or stroke cause death during follow-up period. The subjects with history of stroke at baseline were excluded when we defined incident stroke and performed the survival analysis for the subsequent outcomes. Stroke was defined according to the WHO Multinational Monitoring of Trends and Determinants in Cardiovascular Disease criteria,24 as rapidly developing signs of focal or global disturbance of cerebral function, lasting more than 24 hours (unless interrupted by surgery or death) with no apparent non-vascular causes. Haemorrhagic stroke was defined as stroke cases with diagnosis of subarachnoid haemorrhage or intracerebral haemorrhage and ischaemic stroke was defined as stroke cases with diagnosis of thrombosis or embolism. Transient ischaemic attack and chronic cerebral vascular disease were excluded. For all participants reporting possible diagnoses or death, all available clinical information was collected including medical records and death certificates. All materials were independently reviewed and adjudicated by the end-point assessment committee.
Statistical analysis
All statistical analyses were performed with SPSS V.23.0 software. Continuous variables were reported as mean values±SD, and categorical variables were represented as numbers and percentages. Differences among categories were evaluated using the t-test, non-parameter test, χ2-test or Fisher’s exact test as appropriate. Kaplan-Meier estimates were adopted to calculate the cumulative incidence of stroke and stroke-free survival for each group, and log-rank test was used to compare the differences in estimates. Cox proportional hazards models were used to identify the associations of LAE, LAD and LAD/BSA with the incidence of stroke and stroke cause mortality with HRs and 95% CIs calculated after adjusting the potential confounders. As restricted cubic spline makes it possible to characterise the flexible and visible dose–response relationship between an exposure and outcome,25 this method was used to depict the dose–response associations between LAD, LAD/BSA and the risk of stroke or stroke cause mortality based on logistic regression models. The adjusted covariates included age, gender and other potential confounders. All tests were two-tailed and p<0.05 indicated statistical significance.
Patient and public involvement
No patients were involved in setting the research questions or outcome measures, and they were not involved in the design or performance of this study. No plans were set to disseminate the research results to study participants.
Results
Baseline clinical and echocardiographic characteristics
In this study, there were 8915 subjects with normal LAD and 1126 ones with LAE. Except for LVEF and statin use, there were statistical differences in the distribution of baseline clinical and echocardiographic parameters between two groups (all p<0.05), as shown in table 1. Participants with LAE had a significantly increased prevalence of hypertension and AF compared with normal LAD group as would be anticipated.
Baseline clinical and echocardiographic characteristics of study participants stratified by LAE status
Incidences of clinical outcomes
During a median follow-up of 4.66 years, 336 participants (3.5%) developed new onset stroke and 106 subjects (1.1%) died from stroke in the total cohort. The incident rates of overall stroke and mortality were statistically higher in participants with LAE than those in normal LAD group (all p<0.05) (table 2). The total population was further subdivided according to gender and stroke type. Compared with normal LAD group, incidence of stroke in either male or female subjects with LAE was significantly higher, but only male subjects with LAE had higher incidence of stroke cause mortality (all p<0.05). Incident ischaemic stroke as well as haemorrhagic stroke cause mortality was significantly more frequent in participants with LAE (all p<0.05) (table 2). Additionally, stratified analyses by AF showed that only three subjects had incident stroke and stroke cause death in AF participants respectively, and LAE group displayed a significantly increased stroke incidence when compared with normal LAD group in the population without AF (p<0.01) (online supplemental table 1).
Supplemental material
Incidence of stroke and stroke cause mortality in study participants
LA size for predicting stroke and stroke cause mortality
Kaplan-Meier survival estimates revealed that participants with LAE had higher cumulative stroke incidence and worse stroke-free survival than those with normal LAD in overall population (figure 1). There was an association between LAE and stroke incidence in men or women, and a similar trend was observed for ischaemic stroke incidence (all p<0.01) (figure 2). As described, LAE was significantly associated with worse stroke-free survival in men and ischaemic subtype during follow-up (all p<0.05) (figure 3).
Kaplan-Meier survival curves for stroke incidence (A) and stroke-free survival (B) in overall population according to LA size. LA, left atrial; LAE, LA enlargement.
Kaplan-Meier survival curves for stroke incidence in men (A) and women (B), and ischaemic (C) and haemorrhagic (D) stroke incidence according to LA size. LA, left atrial; LAE, LA enlargement.
Kaplan-Meier survival curves for stroke-free survival in men (A) and women (B), and ischaemic (C) and haemorrhagic (D) stroke-free survival according to LA size. LA, left atrial; LAE, LA enlargement.
However, in the multivariable model after adjusting the confounding factors, the association between LAE and the risk of stroke or stroke cause mortality was no longer significant, as presented in table 3 and online supplemental table 2. When LAD and LAD/BSA were treated as continuous variables, per 1 SD increase in LAD or LAD/BSA was significantly associated with an elevated risk of stroke (LAD: HR=1.20, 95% CI 1.08 to 1.33, p<0.001; LAD/BSA: HR=1.22, 95% CI 1.11 to 1.35, p<0.001) and stroke cause mortality (LAD: HR=1.27, 95% CI 1.08 to 1.50, p<0.01; LAD/BSA: HR=1.41, 95% CI 1.20 to 1.65, p<0.001) in overall population (table 3). Further stratified analyses showed that LAD or LAD/BSA was closely related to the incidence of stroke and stroke cause mortality in both male and female subjects (all p<0.05) (table 3). After the adjustment for the potential confounders, a relationship of LAD with incident ischaemic stroke, and a significant association between LAD/BSA and the risk of both ischaemic stroke and its cause death were observed, while LAD and LAD/BSA were correlated with an increased risk of haemorrhagic stroke and its mortality (all p<0.05) (table 3). When the participants with AF were excluded, the predictive value of LAD and LAD/BSA for incident stroke and stroke cause mortality remained significant in the subjects without AF (all p<0.01) (online supplemental table 2).
LA size for the prediction of stroke and stroke cause mortality during follow-up
Dose–response analyses of the associations between LAD, LAD/BSA and the risk of stroke or stroke cause mortality
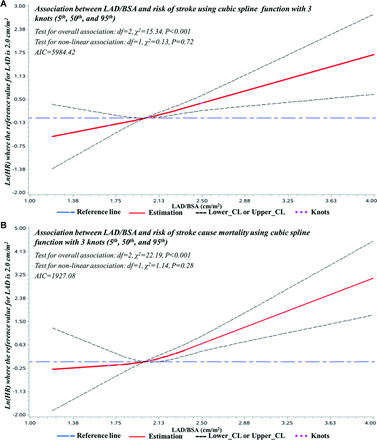
After the adjustment for age, gender, BSA, smoking, drinking, heart rate, history of coronary heart disease (CHD), history of AF, history of heart failure (HF), history of heart valve diseases, hypertension, diabetes, medication for hypertension and diabetes, dyslipidaemia, estimated glomerular filtration rate (eGFR), statin use, aspirin use, LVMI, LVEF, E/e’ as appropriate, the dose–response curves indicated linear associations between LAD, LAD/BSA and stroke risk in the general population (for LAD, test for overall association: p<0.001; test for non-linear association: p=0.80, Akaike information criterion(AIC)=5984.42; for LAD/BSA, test for overall association: p<0.001; test for non-linear association: p=0.72, AIC=5984.42) (figures 4 and 5) (online supplemental tables 3 and 4). Similarly, significant linear dose–response associations between LAD, LAD/BSA and the risk of stroke cause death were observed (for LAD, test for overall association: p<0.001; test for non-linear association: p=0.12, AIC=1927.08; for LAD/BSA, test for overall association: p<0.001; test for non-linear association: p=0.28, AIC=1927.08) (figures 4 and 5) (online supplemental tables 3 and 4).
Adjusted dose–response associations between LAD and the risk of stroke (A) and stroke cause mortality (B). Adjusted for age, gender, BSA, smoking, drinking, heart rate, history of CHD, history of AF, history of HF, history of heart valve diseases, hypertension, diabetes, medication for hypertension and diabetes, dyslipidaemia, eGFR, statin use, aspirin use, LVMI, LVEF, E/e′ as appropriate. The Y-axis indicates the ln(HR) of stroke for any value of LAD compared with the reference values. Dashed lines refer to the 95% CIs. AIC, Akaike information criterion; CL, confidence interval; LAD, left atrial diameter; BSA, body surface area; CHD, coronary heart disease; AF, atrial fibrillation; HF, heart failure; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction.
Adjusted dose–response associations between LAD/BSA and the risk of stroke (A) and stroke cause mortality (B). Adjusted for age, gender, BSA, smoking, drinking, heart rate, history of CHD, history of AF, history of HF, history of heart valve diseases, hypertension, diabetes, medication for hypertension and diabetes, dyslipidaemia, eGFR, statin use, aspirin use, LVMI, LVEF, E/e′ as appropriate. The Y-axis indicates the ln(HR) of stroke cause mortality for any value of LAD/BSA compared with the reference values. Dashed lines refer to the 95% CIs. AIC, Akaike information criterion; CL, confidence interval; LAD, left atrial diameter; BSA, body surface area; CHD, coronary heart disease; AF, atrial fibrillation; HF, heart failure; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction.
Discussion
In this large Chinese population-based cohort, we showed a significantly higher incidence of stroke and stroke cause mortality in LAE group compared with normal LAD group in the overall and stratified analyses. Interestingly, our study further demonstrated that LAD and LAD/BSA rather than LAE were possible predictors of incident stroke and its mortality in the general population and specific subgroups, and there could be linear dose–response associations between LAD, LAD/BSA and the risk of stroke and stroke cause mortality.
Enlarged LA size appears to be increasingly regarded as a prognostic biomarker for thromboembolic and cardiovascular events.26 27 A number of population-based studies tried to evaluate the role of LA size in predicting stroke, obtaining controversial results. Some studies supported the association between LA size and stroke risk,4 8 9 whereas others did not.10–13 Froehlich et al conducted a meta-analysis to reveal that indexed LAD could be an important predictor of stroke without adjustment for cardiovascular risk factors.26 A recent systematic review involving six cohorts concluded that LAE was related to an increased and graded risk of stroke independent of age, sex, hypertension and diabetes.2 In our study, when LAD was dichotomised based on sex-specific partition values, subjects with LAE exhibited elevated incident rates of stroke and its mortality in the overall and stratified comparisons. The Kaplan-Meier curves revealed that LAE was significantly associated with higher stroke incidence in male, female and ischaemic subtype, as well as worse stroke-free survival in male and ischaemic stroke. Of note, our multivariate analysis did not confirm LAE as an independent predictor for stroke and long-term survival. The reasons for these discordant results might be the ethnicity-related differences, study design heterogeneity, disparities in measurements, indexations and cut-off points to define LAE, and diverse adjustment factors. Another possibility is that the link between LAE and the risk of stroke and its cause death is mediated by a combination of other clinical covariates.28 29
When LA size was considered continuously, Cox regression analysis indicated that per 1 SD increase in LAD or LAD/BSA was significantly correlated with an increased risk of stroke and stroke cause mortality in overall population after adjusting the potential confounders. The associations between LAD, LAD/BSA and the risk of stroke and its mortality were further verified by the dose–response analyses. Importantly, using the drawn dose–response curves, we could calculate the risks of developing stroke and subsequent mortality at any specific LAD or LAD/BSA values, which would facilitate risk assessment and individualised management strategies.30 Above findings implied that close monitoring of echocardiographic LAD increment might add predictive information in the evaluation of stroke and its mortality, which needed to be validated through replication in other populations. However, Mosquera et al found that LAD/BSA raised the risk of stroke in 50.7% per 1 cm/m2 increment in bivariate analysis, which association disappeared after multivariate regression.31
Sexual differences in the association between LA size and stoke risk are of huge interest in current research.32 33 Some studies suggested that LAE could be considered as a potential risk factor for ischaemic stroke only in men, but others reported this association only in women.14 In our research, there were no obvious sex differences regarding the correlation analysis between LAD, LAD/BSA and incident stroke and stroke cause mortality. Furthermore, we found that LAD and LAD/BSA could increase the risk of incident ischaemic stroke or its mortality. Interestingly, the present study also provided new evidence about such a relationship of LAD and LAD/BSA with the incidence of haemorrhagic stroke and its mortality, which has not been reported previously. The underlying mechanisms are not fully elucidated, but some hypotheses have been proposed, which might partially account for above associations. First, enlarged LA size may be a surrogate marker for cardiac and/or vascular weakening and damage, which are correlated with increased risk of stroke and mortality.11 34 Second, LA dilation may promote blood stasis, which in turn predisposes to thrombus formation and the potential for the embolisation, and carries its association with higher risk of ischaemic stroke.17 35 36 Third, the most common cause of haemorrhagic stroke is hypertension, and LAE is often viewed as a consequence of exposure to hypertension burden.5 37 Additionally, oral anticoagulation therapy may contribute to the bleeding tendency in haemorrhagic stroke.38 39 Our findings have possible clinical implications for stroke risk stratification, screening and stroke prevention.
Several limitations should be addressed. First, this was a single centre study and we enrolled subjects capable of receiving echocardiography, both of which perhaps caused selection bias. Second, although we adjusted for potential risk factors in the multivariate analysis, there might be residual confounders. Third, there was the lacking information on detailed stroke type (eg, cardioembolic) and stroke severity. Finally, the limited number of events due to short follow-up time might restrict the study conclusions. Then, larger multi-centre prospective researches with longer periods of follow-up are deserved to confirm our findings.
Conclusion
Our prospective cohort study found that subjects with LAE were more likely to display an elevated incidence of stroke and poor survival prognosis than those with normal LAD. LAD and LAD/BSA were possible predictors for incident stroke and subsequent mortality in the general population and specific subgroups. Echocardiographic LA size, especially LAD and LAD/BSA, holds promise as a valuable means of predicting stroke and stroke cause mortality, and might be useful in developing diagnostic and preventive therapeutic strategies.
Acknowledgments
We would like to thank Professor Liqiang Zheng for his help with data collection and data management.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors TL participated in echocardiographic measurements and wrote the manuscript. GL conducted statistical analyses. XG assisted with critical revision of manuscript for intellectual content. ZL coordinated data collection and subjects’ follow-up. JY contributed to echocardiographic analyses and readings. YS was responsible for the study concept and design. All authors have read and approved the final manuscript.
Funding This study was supported by grants from the National Key Research and Development Programme of China (2017YFC1307600), National Key Research and Development Programme from the Ministry of Science and Technology of China (2018YFC1312403), National Natural Science Foundation of China (82001828) and Natural Science Foundation of Liaoning Province (2020-BS-102).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by the Ethics Committee of China Medical University (Shenyang, China) (2018194). Written informed consent was obtained from all participants.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.