Article Text
Abstract
Objective To examine the cost-effectiveness of nurse-led stroke aftercare addressing psychosocial outcome at 6 months post stroke, compared with care-as-usual.
Design Economic evaluation within a comparative effectiveness research design.
Setting Primary care (2016–2017) and community settings (2011–2013) in the Netherlands.
Participants Persons who suffered from ischaemic or haemorrhagic stroke, or a transient ischaemic attack and were discharged home after visiting the emergency department, hospitalisation or inpatient rehabilitation.
Interventions Nurse-led stroke aftercare at 6 months post stroke addressing psychosocial functioning by providing screening, psycho-education, emotional support and referral to specialist care when needed. Care-as-usual concerned routine follow-up care including secondary prevention programmes and a consultation with the neurologist at 6 weeks post stroke.
Primary and secondary outcome measures Main outcome measure of cost-effectiveness was quality-adjusted life years (QALYs) estimated by the quality of life measured by the five-dimensional, three-level EuroQol. Costs were assessed using a cost-questionnaire. Secondary outcomes were mood (Hospital Anxiety and Depression Scale) and social participation (Utrecht Scale for Evaluation of Rehabilitation-Participation) restrictions subscale.
Results Health outcomes were significantly better in stroke aftercare for QALYs (Δ=0.05; 95% CI 0.01 to 0.09) and social participation (Δ=4.91; 95% CI 1.89 to 7.93) compared with care-as-usual. Total societal costs were €1208 higher in stroke aftercare than in care-as-usual (95% CI −€3881 to €6057). Healthcare costs were in total €1208 higher in stroke aftercare than in care-as-usual (95% CI −€3881 to €6057). Average costs of stroke aftercare were €91 (SD=€3.20) per person. Base case cost-effectiveness analyses showed an incremental cost-effectiveness ratio of €24 679 per QALY gained. Probability of stroke aftercare being cost-effective was 64% on a €50 000 willingness-to-pay level.
Conclusions Nurse-led stroke aftercare addressing psychosocial functioning showed to be a low-cost intervention and is likely to be a cost-effective addition to care-as-usual. It plays an important role by screening and addressing psychosocial problem, not covered by usual care.
- stroke
- health economics
- health services administration & management
- primary care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study examined nurse-led stroke aftercare, an addition to routine follow-up care, specifically aimed at the psychosocial outcome by screening, psycho-education and emotional support, and referral when needed.
A full economic evaluation using a comparative effectiveness research design was conducted to evaluate the cost-effectiveness of stroke aftercare compared with care-as-usual.
Inevitable differences in research design and time of recruitment between observational cohorts may have had an impact on the uncertainty regarding the cost-effectiveness of stroke aftercare.
Ideally, the five level version of the quality of life measured by the five-dimensional was used because of less ceiling effects and greater discriminatory capabilities than the three-level version.
Introduction
Stroke can cause people to suffer from long-lasting physical problems,1 cognitive impairment2 and emotional difficulties3 which makes it one of the most disabling chronic conditions worldwide.4 The consequences of stroke can negatively affect the level of social participation5 6 and quality of life (QoL).7 8 Moreover, stroke has a substantial economic impact on society.9
Worldwide, the average national costs of stroke are 3%–4% of the total healthcare expenditures.10 Stroke costs are expected to rise in the future because of increasing stroke prevalence rates, attributed to a growing and ageing population, and higher survival rates because of improved acute care.4 Persons with stroke increasingly reside in the community,11 shifting the costs of inpatient care to costs arising from community services.10 At this point in time, sustainable healthcare for stroke is essential in managing the (increasing) economic impact of stroke on society.
Moreover, the healthcare system needs to be improved to give persons with stroke access to appropriate care and successfully navigate the healthcare system.12 The current routine follow-up care after stroke, or stroke care pathway, is primarily focused on secondary prevention and neurological symptoms in the first weeks post stroke,13 rather than on psychosocial functioning.14 The stroke stepped-care model, proposed by Kneebone, states that most persons with stroke experience mild mood problems, in need of psycho-education and emotional support.15 Furthermore, the model argues that only a minority needs more extensive, specialised care.14 15 Appropriate aftercare should pay attention to the variability in symptom severity and fit the needs of individual persons with stroke.16
Effective aftercare addressing psychosocial outcome after stroke is scarce,17 and when effective, include extensive community care models such as home-based rehabilitation18 19 which do not align with the stepped-care model. Although provided in a cardiac-arrest population with hypoxic brain damage, the content of the brief nurse-led intervention designed by Moulaert et al20 aligns with the stepped-care model and can be used as an example in designing appropriate aftercare for persons with stroke. Nurses provided care on an individual level through cognitive and emotional screening, psycho-education and referral to specialist care when needed. The intervention started directly after discharge from the hospital and consisted of only one or two face-to-face meetings at home or in an outpatient clinic.20 This nurse-led intervention was shown to be feasible, clinically effective as well as cost-effective.21 22
Primary care plus (PC+) is a new form of healthcare that could serve as the appropriate setting to provide aftercare. PC+ aims to reduce healthcare costs by substituting non-acute hospital care to primary care while ensuring specialist knowledge of the disease. PC+ is implemented in the southern part of the Netherlands in which different medical specialists, as well as trained nurses, perform consultations.23 24 Nurses are less costly to employ and their care leads to higher patient satisfaction.25 26 An intermediate evaluation of PC+ showed that healthcare costs per patient were reduced, patient satisfaction increased and health outcome was comparable to outpatient hospital care.27
New nurse-led aftercare addressing psychosocial functioning for persons with stroke is added to our regional stroke care pathway within the PC+ context, in which screening, psycho-education, emotional support and referral is provided. This study aimed to examine the cost-effectiveness of this new PC+ nurse led stroke aftercare at 6 months post stroke compared with care-as-usual from a societal perspective and with a 9-month time horizon.
Methods
Design and setting
This study concerned a full economic evaluation using a comparative effectiveness research design to evaluate stroke aftercare compared with care-as-usual. The study was performed according to the Dutch guidelines for economic evaluations in healthcare28 and is reported according to the Consolidated Health Economic Evaluation Reporting Standards guidelines.29
The stroke aftercare cohort study concerned a single centre, prospective, observational design in which persons were recruited at stroke aftercare in the Netherlands (November 2016–December 2017), approximately 6 months post stroke, and followed for 6 months after their visit.
The care-as-usual cohort is part of the multicentre, prospective, observational, Restore4stroke cohort study wherein persons with stroke were recruited from six general hospitals in the Netherlands (March 2011–March 2013) and were followed for 2 years post stroke.6 30
Participants
Persons were invited to visit stroke aftercare and the cohort study if they (>18 years) suffered from clinically confirmed ischaemic or haemorrhagic stroke, or a transient ischaemic attack (TIA) with hospitalisation and who were discharged home after visiting the emergency department, hospitalisation or inpatient rehabilitation. Those not invited to stroke aftercare were discharged directly to a nursing home or outside of the region Maastricht-Heuvelland. Persons were excluded from the stroke aftercare study if they had insufficient command of the Dutch language or no legal competence.
Persons were included in the Restore4stroke care-as-usual cohort study if they had a clinically confirmed diagnosis of either ischaemic or haemorrhagic stroke within the past 7 days as confirmed by the neurologist. Persons were excluded when they had a comorbid condition which was anticipated to interfere with study outcomes such as neuromuscular diseases, premorbid Barthel Index Score <18 indicative of premorbid dependency in activities of daily life, insufficient command of the Dutch language to understand and complete the questionnaires or premorbid cognitive decline as indicated by a score ≥1 on the hetero-anamnesis list cognition. Persons were only included for analyses in this study if they completed at least two full assessments and were living at home during the full study period.
Interventions
Stroke aftercare is current practice and part of the stroke care pathway in the Maastricht-Heuvelland region, the Netherlands. The aims of stroke aftercare are to screen for potential physical, cognitive and emotional problems in daily life, to provide the person with stroke with psycho-education and emotional support, and to refer the person to further specialised healthcare professionals when needed. Persons with stroke and their caregiver receive an invitation for a consultation at stroke aftercare at discharge from the hospital. The consultation is planned at approximately 6 months post stroke in a PC+ centre and is led by a hospital nurse specialised in neurology. Two weeks prior to the consultation, the persons with stroke and their caregiver are asked to complete questionnaires by mail which serve as a source of information in the nurse consultation. The consultation with the nurse takes up to a maximum of 45 min. A follow-up consultation at the stroke aftercare can be planned if the nurse judges this as necessary. In addition to the consultation, 45 min of administration time is included in which the nurses send and process the questionnaires beforehand, report the consultation, consult the general practitioner and if necessary, arrange a referral to a specialised healthcare professional and/or schedule a follow-up consultation.
In the care-as-usual cohort persons were enrolled in secondary prevention programmes after discharge from the hospital as stated by Dutch guidelines31 and received an invitation to consultation with the neurologist at 6 weeks post stroke. Practice variation exists in secondary prevention programmes13 but no further structural follow-up took place afterwards. Resource use and cost data of the Restore4stroke cohort study was earlier described by van Eeden et al6.
Procedure
Those who visited stroke aftercare at PC+ were invited to participate in the stroke aftercare study. At consultation, the nurse gave basic study information and asked whether the person with stroke was interested in participating. If interested, the persons’ contact information was sent to the researcher who provided the person with additional information via telephone. Those willing to participate were sent a written information letter, an informed consent form and the first questionnaire regarding costs and QoL. After written informed consent was obtained, the mood and participation questionnaires, part of stroke aftercare and demographic and medical information were collected from hospital files. The questionnaires administered at the time of stroke aftercare, approximately 6 months post stroke, are regarded as T1. Subsequent questionnaires were sent at 3 months (T2) and 6 months after the stroke aftercare consultation (T3).
Persons eligible for the Restore4stroke cohort study were informed by a nurse practitioner or trial nurse. Written informed consent was obtained after which demographic and stroke-related information was gathered from medical charts by the nurse. The original Restore4stroke study is described in more detail elsewhere.6 The 6-month and 12-month post stroke assessments from Restore4stroke were selected for comparison, matching, respectively the T1 and T3 assessment of the stroke aftercare study.
Time horizon
The total period in which healthcare utilisation was assessed differed between stroke aftercare and care-as-usual. In the stroke aftercare study, healthcare utilisation was assessed 3 months in retrospect at each time point; covering a 9-month time horizon. In the care-as-usual study, healthcare utilisation was assessed 4 months in retrospect at T1 and 6 months at T3; covering a total period of 10 months (figure 1). We corrected for this 1 month difference in period by applying a factor 5/6 on the cost data at T3 (6–12 months post stroke) of the care-as-usual cohort (figure 1). This way we ensured a conservative approach as lower costs can be expected on the long-term relative to short-term costs, which for example, include rehabilitation costs.
Time horizon and correction of time period of care-as-usual in order to align with stroke aftercare.
Outcome measures
The main outcome measure for the cost-effectiveness analyses was QoL measured by the five-dimensional, three-level EuroQol (EQ-5D-3L).32 Dimensions assessed are mobility, self-care, usual activities, pain/discomfort and anxiety/depression which are scored on three levels, ‘no problems’, ‘some problems’ and ‘extreme problems’. UK tariffs33 were used to transform scores into utilities which range from −0.59 (worse than dead) to 1 (full health).32 34 Utilities using Dutch tariffs range from −0.33 to 1.34 Quality-adjusted life years (QALYs) were calculated from utilities by using the area under the curve method. The EQ-5D-3L was administered at T1 and T3 which results in a 6 months assessment period. We extrapolated the 6-month QoL assessment period to equal the 9 months of costs assessment, resulting in a maximum QALY of 0.75.
Secondary outcome measures concerned mood problems and experienced restrictions with participation. The Hospital Anxiety and Depression Scale (HADS) consists of 14 items assessing anxiety and depression scored on a four-point scale (0–3),35 with a total score ranging from 0 to 42. The subdomains anxiety and depression both consist of seven items with a range of 0–21. The total and subdomain scores were reversed with higher scores indicating less severe mood problems. The restrictions scale of the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P) consists of 11 items which are scored on a four-point scale, from no problems at all to not possible to perform that activity. Scores are converted to a 0–100 scale in which higher scores indicate less experienced problems with participation.36
Resource use and costs
A societal perspective was used incorporating all relevant costs, irrespective of who bears the costs. The used methods were identical across cohorts. Resource use data was obtained using a bottom-up approach. A 14-item self-report cost questionnaire was used in which persons indicated type and volume of healthcare they received as well as non-healthcare resource use.6 Valuation of healthcare and non-healthcare unit costs are displayed in online supplemental appendix 1. Costs were calculated by multiplying resource use by unit price. Costs of medication (prescribed as well as over-the-counter drugs) were valued according to the market prices in December 2018 (including 6% tax).37 Pharmacist costs were included (€7 per prescribed medication). When uncertainty occurred with regard to costing, a conservative approach was used (ie, lowest price). Costs of a consultation at stroke aftercare were calculated using a bottom-up approach. This included the hourly wages of the specialised nurses times the consultation duration (1.5 hours in total, including administration time) and the costs of printing and sending questionnaires. A 30% rate was applied to account for overhead costs such as housing costs.28 Training costs were not applicable. Stroke aftercare costs were not applicable to care-as-usual. Informal care was valued by using standard cost prices based on the average hourly wages of professionals performing the same tasks (ie, domestic help28 38). The human capital approach was used to calculate productivity costs, in which lost productivity hours are multiplied by the mean hourly wage, corrected for sex.39 All costs were indexed to the reference year 2018 using consumer price index rates.40 No discounting of costs was needed, as the study period did not exceed 1 year.
Supplemental material
Handling of censored data
Multiple imputation was used to replace missing values, using IBM SPSS V.25. Age, gender, educational level, stroke severity and hemisphere were used as predictors of missing values. Five imputed datasets were generated of which average values were used for statistical analyses.
Statistical analyses
The stroke aftercare cohort was compared with the care-as-usual cohort on demographic and stroke-related variables using independent-samples t-tests and Pearson χ2 tests. The regression-based adjustment of Manca et al41 was applied in calculating QALYs to account for potential bias resulting from baseline differences between cohorts.41 42 Likewise, the T3 scores of the HADS and USER-P were corrected for T1 using regression-based adjustment as described by Vickers and Altman.43 Independent samples t-tests were used to examine differences between cohorts at T1 and T3 in utility scores, QALYs, HADS total and USER-P restrictions. Paired samples t-test were used to examine change over time within each cohort in the same domains. The minimal clinical important difference (MCID) was calculated as the half of the baseline SD per outcome measure.44
Differences in mean resource use between cohorts was examined using independent samples t-test per cost category. Cost data were non-parametrically bootstrapped (1000 replications) to examine the differences in total costs between cohorts in the specified period and changes over time for the stroke aftercare specifically. T2 and T3 were separately compared with T1 to examine costs over time in stroke aftercare using bootstrap analyses.
An incremental cost-utility ratio (ICUR) was calculated by dividing incremental costs by incremental QALYs. An incremental cost-effectiveness ratio (ICER) was calculated by dividing incremental costs by incremental effects of the HADS and USER-P. ICERs and ICURS were bootstrapped (5000 replications) to account for skewed data.45 Cost-effectiveness planes were presented through bootstrapped pairs of cost-effectiveness and cost-utility.
Finally, a cost-effectiveness acceptability curve (CEAC) was calculated to describe the probability of the stroke aftercare being cost-effective in comparison to care-as-usual.46 This CEAC includes the amount of money the society is willing to pay (WTP) to gain one unit of effect (one QALY here). The WTP threshold in the Netherlands for one QALY is €50 000 (2015).47 P values of 0.05 and CIs of 95% were used for significance testing. Statistical analyses were performed using IBM SPSS V.25 and Microsoft Excel 16 for bootstrapping.
Sensitivity analyses
Five one-way sensitivity analyses were performed to check whether base-case assumptions influenced study results. First, persons who suffered from TIA were excluded from the stroke aftercare cohort, as it can be argued that these persons follow a different healthcare trajectory than persons who suffered from stroke and because TIA cases were not included in the care-as-usual cohort. Second, the correction in period in care-as-usual cohort was applied to T1, instead of to T3, as it can be argued that the most expensive care (inpatient rehabilitation) is consumed in the first months post stroke and need correction for comparison (figure 1). Third, stroke aftercare costs were increased by 50% to simulate the situation when an advanced practice registered nurse would lead stroke aftercare. Fourth, Dutch-tariffs were used as small differences exist in which domains are valued more influencing on QoL between countries.33 Finally, the HADS domains of anxiety and depression were examined separately.
Patient and public involvement
This research was designed and performed without active patient or public involvement.
Results
Sample
In total, 84 persons with stroke were included for analyses in the stroke aftercare cohort, and 306 in the care-as-usual cohort (figure 2). Characteristics of both cohorts are displayed in table 1. Patients in the stroke aftercare cohort showed higher stroke severity scores (p<0.05) and larger ischaemic and TIA proportions than care-as-usual (p<0.05).
Flow of persons with stroke through stroke aftercare study and care-as-usual study.
Characteristics of stroke aftercare versus care-as-usual
Health outcome
Stroke aftercare showed significantly higher mean utility scores than care-as-usual at both T1 and T3 (Δ=0.08; 95% CI 0.02 to 0.14 and Δ=0.05; 95% CI 0.01 to 0.09, respectively). No significant changes over time were observed in utility scores in stroke aftercare (Δ=−0.01; 95% CI −0.02 to 0.01), while care-as-usual showed a significant increase of 0.02 over time (95% CI 0.01 to 0.03) (table 2). The MCID was set at 0.12 increase in utility score, which was not observed for either cohort. QALYs were shown to be 0.59 (SD=0.13) in stroke aftercare and 0.54 (SD=0.16) in care-as-usual which were statistically different (Δ=0.05; 95% CI 0.01 to 0.09).
Quality of life, emotional functioning and participation outcome over time per cohort
HADS total score did not differ significantly between cohorts at T1 (Δ=−0.02; 95% CI −1.8 to 1.76) and T3 (Δ=0.04; 95% CI −1.27 to 1.35). No significant changes were observed over time in HADS total score, both in stroke aftercare (Δ=0.20; 95% CI −0.24 to 0.64) and care-as-usual (Δ=0.14; 95% CI −0.08 to 0.35). The MCID was set at 3.68 increase in HADS total score, which was not observed for either cohort.
USER-P restrictions scores were significantly higher in stroke aftercare than in care-as-usual at T1 (Δ=5.46; 95% CI 1.15 to 9.77) and T3 (Δ=4.91; 95% CI 1.89 to 7.93). Within stroke aftercare, no significant change was observed over time for USER-P restrictions (Δ=0.99; 95% CI −0.11 to 2.09), while a significant increase over time was observed in care-as-usual (Δ=1.54; 95% CI 0.86 to 2.21). The MCID was set at 9.80 increase in USER-P restrictions score, which was not observed for either cohort.
Total societal costs
Mean total societal costs over 9 months were higher in the stroke aftercare cohort than in the care-as-usual cohort, but not significantly different (Δ=€1208; 95% CI −€3881 to €6057) (table 3). In the stroke aftercare cohort over time, mean societal costs were highest at T1 (€7003) in comparison to T2 (€4549) and T3 (€6131). Societal costs at T2 were significantly lower than at T1 in stroke aftercare (Δ=−€2455; 95% CI −€4809 to −€66) (online supplemental appendix 2).
Supplemental material
Resource use and costs in euros (€) of persons who received stroke aftercare and care-as-usual over the total period of 9 months
Healthcare and non-healthcare costs
The mean total healthcare costs were lower in the stroke aftercare cohort than in the care-as-usual cohort but were not significantly different (Δ=−€1695, 95% CI −€3946 to €514). Mean resource use of overnight stays at a rehabilitation clinic were significantly lower in stroke aftercare than in care-as-usual (Δ=−3.71, 95% CI −5.44 to −1.99). Significant lower costs were observed for the stroke aftercare cohort in rehabilitation clinic costs as well as nursing home stay, compared with care-as-usual (Δ=−€1770; 95% CI −€2719 to −€1043 and Δ=−€119; 95% CI −€254 to −€28, respectively). Significant higher healthcare costs in stroke aftercare were observed regarding general practitioner and medication (Δ=€40, 95% CI €1 to €86 and Δ=€79, 95% CI €19 to €143, respectively). The average costs of stroke aftercare were €91 (SD=€3.20) per person (including follow-up consultations). In the stroke aftercare cohort, mean healthcare costs significantly decreased from T1 to T2 (Δ=−€1289, 95% CI −€2461 to −€171) and increased again to T3. T3 did not significantly differ from T1 (Δ=−€890, 95% CI −€2192 to €254). The percentage healthcare costs of total societal costs decreased from 43% at T1, to 39% and 35% at T2 and T3, respectively (online supplemental appendix 2).
The mean total non-healthcare costs were higher in the stroke aftercare cohort than in the care-as-usual cohort, but the mean difference was not significantly different between cohorts (Δ=€2851, 95% CI −€467 to €6485). Significantly higher mean resource use (Δ=140.74, 95% CI 17.58 to 263.91) and significantly higher unit costs were observed for informal care in the stroke aftercare cohort than in care-as-usual (Δ=€2048, 95% CI €403 to €3946). The mean non-healthcare costs did not significantly change in the stroke aftercare cohort from T1 to T2 (Δ=−€1291, 95% CI −€3147 to €499) and T3 did not differ from T1 (Δ=−€58, 95% CI −€1962 to €1917). The percentage non-healthcare costs increased from 57% at T1 to 61% and 65% at T2 and T3, respectively (online supplemental appendix 2).
Cost-utility analysis
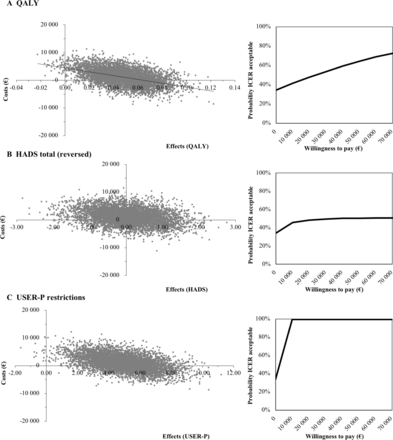
The base case ICUR analysis showed that the average QALY outcome was 0.59 for stroke aftercare and 0.54 for care-as-usual which means that stroke aftercare gained 0.05 more QALYs than care-as-usual (table 4). This gain in QALYs after stroke aftercare, combined with, on average, more societal costs (∆=€1171) resulted in an ICER of €24 679. Bootstrapped pairs are displayed in figure 3A, where 33% of the pairs were in the dominant South-East (SE) quadrant indicating more effects, lower costs and 66% was in the North-East (NE) quadrant indicating more effects, more costs. Using the €50 000 WTP threshold, there is a probability of 64% that stroke aftercare will be cost-effective (figure 3A).
Base-case and sensitivity analyses of stroke aftercare vs care-as-usual, incremental cost-effectiveness ratios and plane distributions
Cost-effectiveness planes displayed on the left and cost-effectiveness acceptability curves displayed on the right per outcome measure. HADS, Hospital Anxiety and Depression Scale; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years; USER-P, Utrecht Scale for Evaluation of Rehabilitation-Participation.
Cost-effectiveness analyses
The base case ICER analyses showed that the average HADS total outcome in stroke aftercare was minimally different from care-as-usual (∆=0.04). Combined with higher societal costs this resulted in an ICER of €27 710 (table 4). Figure 3B shows the bootstrapped pairs of the HADS total, in which 30% of the pairs are in the dominant SE quadrant and 36% in the inferior North-West (NW) quadrant, indicating less effect and more costs. The USER-P in stroke aftercare was higher than in care-as-usual (∆=4.91) which, combined with higher societal costs, resulted in an ICER of €238 (table 4).Figure 3C shows the bootstrapped pairs of the USER-P, in which 34% of the pairs are in the dominant SE quadrant and 66% in the NE quadrant, indicating more effects and more costs with stroke aftercare.
Sensitivity analyses
Overall, the five one-way sensitivity analyses confirm the findings of the base case analyses as displayed in table 4. The exclusion of TIA cases in the first sensitivity analyses showed minor impact on the distribution of ICERs, in favour of more pairs in the dominant SE quadrant for QALYs and USER-P restrictions. More pairs in the inferior NW quadrant was observed for the HADS as effects decreased by excluding TIA cases. Second, regarding the period correction at T1 in care-as-usual (figure 1), results showed an increase in pairs from SE in base-case analyses towards the NE quadrant in all outcome measures because of lower estimated costs in care-as-usual. Increasing the stroke aftercare costs by 50% in the third sensitivity analyses showed a similar distribution of ICERs in comparison to base case analysis. The fourth sensitivity analyses, in which the Dutch tariff for utility calculation was used, showed a similar distribution of ICERs in comparison to base case analysis with the UK tariff. In the final sensitivity analysis, the HADS anxiety and depression subdomains were separately assessed which did not impact the distribution of ICERs.
Discussion
This study aimed to examine the cost-effectiveness of nurse-led stroke aftercare at 6-months post stroke. The base-case utility analyses showed increased QALY and higher societal costs in the stroke aftercare cohort in comparison to care-as-usual. The probability of stroke aftercare being cost-effective was 64% given the WTP in the Netherlands. Nurse-led stroke aftercare was shown to be a low-cost intervention given the mean cost of €91 per person. No additional effects because of stroke aftercare were shown regarding mood problems but social participation outcomes did differ between cohorts at 12-months post stroke in favour of stroke aftercare. Inevitable differences between the observational studies impact the uncertainty regarding cost-effectiveness and prevent conclusive remarks but results suggest that stroke aftercare could be a cost-effective addition to care-as-usual.
This consideration is based on its low associated costs together with its important role in the stroke care pathway. Stroke aftercare addresses psychosocial functioning in a structural manner as recommended by international guidelines,48 which is achieved through the elements of providing screening, information, emotional support and referral when needed. The importance of screening is emphasised by the sensitivity analysis of TIA cases who were shown to have higher costs and greater emotional benefits of stroke aftercare. This observation was rather unexpected as persons with TIAs have lower healthcare costs49 and report higher QoL50 than stroke. Apparently, a proportion of TIA cases need stroke aftercare as well in which screening is crucial.51 Beneficial effects of information provision and emotional support through counselling have been reported earlier in stroke.52 53 Importantly, healthcare consumption and costs did not increase with stroke aftercare which suggests that referral only took place when needed, rather than systematically. This aligns with the stepped-care design of stroke aftercare.14 15
Stroke aftercare is only considered a sustainable intervention when it complies with the theoretical Triple Aim framework of Berwick et al.54 Although the effects were not considered clinically meaningful, ‘improving health of the population’ has been shown in this study because stroke aftercare showed greater QoL and social participation outcomes than care-as-usual. ‘Improving the experience of care’ is supported by findings of the intermediate report of PC+ of which is stroke aftercare a part.27 Healthcare costs were lower in the stroke aftercare cohort and thereby complies with the final aim of the framework: ‘reduced per capita cost of healthcare’. It must be noted that the lower healthcare costs were mostly due to low costs of inpatient rehabilitation following hospital discharge. As it concerned aftercare for community-dwelling persons, we were less interested in subacute care costs and started measuring at 3 months post stroke in the stroke aftercare study. In contrast, the first assessment of care-as-usual started measuring at 2 months (to 6 months) post stroke. It is likely that inpatient rehabilitation after hospital endured beyond 2 months post stroke and was measured for some individuals in the care-as-usual cohort. Correction for the first assessment period in the care-as-usual cohort, and thus inpatient rehabilitation costs post-hospital discharge, resulted in larger cost differences. These results negatively affected the cost-effectiveness of stroke aftercare. Still, considering the Triple Aim as a whole, stroke aftercare may be regarded as a sustainable addition to the stroke care pathway.
Changing healthcare policies should be considered as the recruitment periods of stroke aftercare and care-as-usual differed (2017 and 2012, respectively). On the level of stroke care, new guidelines have been developed which emphasise for example early supported discharge48 resulting in a decreased length of stay at inpatient rehabilitation over time.55 On national level, the welfare state changed to healthcare policies emphasising individual responsibility and non-residential care, along with a restructuring of the financing system in 2015.56 In line changed healthcare policies over time, informal care costs were 21% of the total societal costs in stroke aftercare compared with 10% in care-as-usual.
Strengths and limitations
Strengths of the current study include prospective design of the observational studies, multiple sensitivity analyses, and performing the economic evaluation in line with national28 and international guidelines28 and with preferred methods such as multiple imputation.57 The main limitation concerned the dissimilarity in time horizons of the two observational cohorts which impacted the uncertainty regarding cost-effectiveness. To enable a direct comparison between cohorts, we corrected for the time horizon dissimilarity in base-case analyses and was further examined sensitivity analyses. Moreover, levels of QoL might have been overestimated as a result of the extrapolation of the assessment period to the three preceding months. In general, higher levels of QoL are observed between 6 and 12 months post stroke than in the three preceding months, three to 6 months post stroke.58 However, this correction did not, influence the conclusions as the QALY extrapolation was equal across cohorts. Because of the comparative effectiveness design, we used EQ-5D-3L in the stroke aftercare although it is recommended to use the five-level version of the EQ-5D with less ceiling effects and greater discriminatory capabilities.59 Finally, when historical cohorts are used in a comparative effective design, it is advised to keep differences in recruitment periods to a minimum in order to maximise comparability of cohorts and thereby strengthen conclusions.
Conclusions
The findings in this study showed better outcomes regarding QoL and social participation after nurse-led stroke aftercare than with care-as-usual. Higher costs were observed for stroke aftercare which are likely attributable to changing healthcare policies. Stroke aftercare showed to be a low-cost intervention and results suggest it to be a cost-effective addition to the stroke care pathway. It plays an important role in the stroke care pathway by screening and addressing psychosocial problems, not structurally covered by usual care.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors DPJV, RWHMP, CMvH and MEALK designed the study. DPJV acquired the data. DPJV, GAPGvM and MEALK analysed and interpreted the data. DPJV drafted the manuscript. GAPGvM, RWHMP, CMvH and MEALK advised on preparation of the manuscript. All authors contributed read, edited and approved the final version of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The medical ethics committee of Maastricht University Medical Center (MUMC+) approved the study protocol (16-4-180, October 2016). The Restore4stroke study was approved by all medical-ethical committees of the participating hospitals and the Committee on Research involving Human Subjects of the St. Antonius Hospital in Nieuwegein, the Netherlands (R-10.41A, February 2011). Both studies were performed according to the Declaration of Helsinki’s principles.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.