Article Text
Abstract
Introduction Hepatitis B virus (HBV) related acute-on-chronic liver failure (ACLF) is still a common type of liver failure in China. Therefore, we conduct this multicentre, non-blinded, randomised controlled clinical trial to investigate the efficacy and safety of combination treatment of double plasma molecular adsorption system (DPMAS) and low volume plasma exchange (PE) for patients with HBV related ACLF.
Methods and analysis A total of 200 patients with HBV related ACLF in the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou Eighth People’s Hospital, Nanfang Hospital of Southern Medical University, The Third People’s Hospital of Shenzhen, Xiangya Hospital of Central South University and The First Affiliated Hospital of Anhui Medical University, will be recruited into this trial. Eligible patients will undergo randomisation at a 1:1 ratio to two arms: the control group and the trial group. Patients in control group will receive comprehensive internal medical treatment. Patients in trial group will receive treatment of DPMAS and sequential low volume PE for three times, and comprehensive internal medical treatment. Clinical safety will be assessed by the analysis of adverse events (AEs) and laboratory tests. The primary efficacy outcome will be the incidence of unfavored events including death, liver transplantation and treatment abandonment. The secondary efficacy outcome will be the model for end-stage liver disease score variation. All evaluations will be performed at baseline, and 4, 8, 12, 24, 36, 48 weeks after enrolment. All AEs will be reported as soon as they are noted during the entire study procedure.
Ethics and dissemination This study was approved by Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (approval no. (2020)02-173-01). The results and conclusions of this clinical trial will be published at academic conferences or in journals.
Trial registration number NCT04597164.
- hepatology
- hepatobiliary disease
- clinical trials
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
It is a multicentre randomised controlled clinical trial in six centres.
The intervention is a new non-cellular artificial liver support systems method that combines double plasma molecular adsorption system with plasma exchange.
The participants are liver failure which is of high mortality rate and lacks of effective treatment.
Introduction
Hepatitis B virus (HBV) infection remains a serious public health problem as there are approximately 257 million chronic HBV infected people worldwide.1 In China, HBV related acute-on-chronic liver failure (ACLF) is the most common type of liver failure due to the high incidence of HBV infection.2 ACLF is a syndrome characterised by acute decompensation of chronic liver disease and organ/system failure(s), with clinical manifestations of gastrointestinal symptoms, jaundice, coagulation dysfunction, hepatic encephalopathy and so on, resulting in a high short-term mortality rate.3 The 90-day mortality of HBV related ACLF is reportedly up to 50%–70%.4 5 There still lack of specific or effective therapies for patients with HBV related ACLF. Although liver transplantation is considered as the only curative therapy for patients with HBV related ACLF,6 it is limited by high cost, organ shortage for transplantation and post-transplantation immune rejection. Without liver transplantation, non-cellular artificial liver support systems (NC-ALSS) are recommended to be an effective therapy for patients with HBV related ACLF by Chinese Medical Association.2 NC-ALSS can temporarily and partially replace the liver functions, which is based on the strong regeneration capacity of hepatic cells.2 It can remove various harmful substances and toxins in the blood, supply essential substances, improve the internal environment, which creates favourable conditions for hepatocellular regeneration and liver function recovery.2 7 It can improve short-term and long-term outcomes of patients with HBV related ACLF.7 8 It can also serve as a bridge to liver transplantation.7
There are various modes for NC-ALSS2: plasma exchange (PE), plasmaperfusion or haemoperfusion, plasma adsorption, haemodialysis, haemofiltration, molecular adsorbent recycling system, continue albumin purification system, fractional plasma separation and adsorption and so on. Among them, PE is the most common treatment mode in China. PE can discard toxin-containing plasma of the patient and replace equal amount of fresh plasma or fresh frozen plasma (FFP) which contains a lot of coagulation factors lacking in liver failure. PE therapy has been shown to improve the hepatic function as well as the short-term and long-term prognosis of patients with HBV related ACLF.7 9 PE therapy needs large amount of fresh plasma or FFP (over 2000 mL per time), that it is limited by plasma supply. Double plasma molecular adsorption system (DPMAS) is a patented technology pioneered by Jafron Biomedical in China. It is one of the NC-ALSS technologies. It uses HA330-Ⅱ Disposable Hemoperfusion Cartridge10 to adsorb medium and large molecular toxins, combined with BS330 Disposable Plasma Bilirubin Adsorption Column11 to adsorb bilirubin and bile acids. DPMAS can adsorb multiple harmful substances without consuming plasma. However, the patient’s coagulation function is affected due to the consumption of coagulation factors and the use of anticoagulants during the DPMAS treatment. PE and DPMAS are well-tolerated and similar in improving 12-week survivals in HBV related ACLF.12
Combination treatment of DPMAS and low volume (about 1000 mL per time) PE is a new way to treat HBV related ACLF in recent days. The combination of the two treatment modes has complementary advantages: maximum removal of bilirubin, inflammatory mediators and other metabolites, and minimum impact on coagulation function to reduce the risk of bleeding. Meanwhile, the combination therapy shares a same set of external circulating blood circuit and plasma separator, which reduces the cost. Guo et al13 compared the effectiveness of PE, DPMAS and PE+DPMAS, and found PE+DPMAS treatment improved the short-term effectiveness of treatment, especially in patients with mild ACLF. Zhong et al14 found that the effectiveness of low-volume PE combined with DPMAS was significantly better than simple PE in early stage of ACLF (83.7% vs 55.6%). Study from Yao et al15 shown that the 28-days survival rate was 62.3% and 72.2% in PE and DPMAS+PE group in patients with HBV related ACLF, respectively. These studies were retrospective, single-centre studies without high-grade evidence. Therefore, this study protocol describes a planned parallel-group clinical trial to explore the advantages and assess efficacy and safety of combination treatment of DPMAS and low volume PE for patients with HBV related ACLF.
Methods and analysis
Study design
This is the protocol for a prospective, multicentre, non-blinded, randomised controlled clinical trial, which was in accordance with the Standard Protocol Items: Recommendations for Interventional Trials checklist (table 1 and online supplemental file 1). The initiating sponsor and coordinator of this clinical trial is the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The other five participants are Guangzhou Eighth People’s Hospital (Guangzhou, China), Nanfang Hospital of Southern Medical University (Guangzhou, China), The Third People’s Hospital of Shenzhen (Shenzhen, China), Xiangya Hospital of Central South University (Changsha, China) and The First Affiliated Hospital of Anhui Medical University (Anhui, China).
Supplemental material
Standard Protocol Items: Recommendations for Interventional Trials diagram
Patients with HBV related ACLF in the above six centres, who meet diagnostic criteria from guidelines,2 6 16 will be recruited in this study. Eligible patients will undergo randomisation at a 1:1 ratio to two arms: the control group (comprehensive internal medical treatment) and the trial group (DPMAS and sequential low volume (1000 mL per time) PE, and comprehensive internal medical treatment). Randomisation will be performed centrally according to a computer-generated number table to ensure that randomisation data are not influenced by any person. In this study, both those giving therapy and the assessors will not be intervention-blinded, participants will not be treatment-blinded.
Eligibility criteria
All the six trial centres will use the same criteria and rigidity for participants inclusion. The inclusion criteria were: (1) aged 18–65 years; (2) positive for HBV surface antigen or positive HBV DNA for longer than 6 months; (3) coagulation disorders (international normalised ratio (INR) >1.5 and ≤2.6; or prothrombin activity (PTA) <40% and ≥20%); (4) severe jaundice (serum total bilirubin (TBIL) ≥10 times upper limit of normal); (5) PLT >50×109/L; (6) have voluntarily signed and dated an informed consent form approved by the ethics committee.
The exclusion criteria were: (1) combined with other active liver diseases; (2) combined with hepatocellular carcinoma or other malignancy; (3) pregnancy or lactation; (4) combined with HIV infection or congenital immune deficiency diseases; (5) combined with severe diabetes, autoimmune diseases; unstable infarction due to cardio-cerebrovascular events; (6) combined with other important organ dysfunctions or transplantation; (7) combined with severe complications including severe infection, gastrointestinal bleeding, hepatic encephalopathy, hepatorenal syndrome; (8) patients cannot follow the research arrangement; (9) patients cannot sign the informed consent; (10) patients cannot follow-up; (11) investigator considering inappropriate.
The cessation criteria were: (1) individual wishes of the participants; (2) occurrence of any treatment associated serious adverse event (SAE); (3) fail to receive treatment due to poor compliance; (4) fail to follow-up; (5) other unpredictable events which lead to termination of the trial.
Interventions
The 100 participants in control group will receive comprehensive internal medical treatment, such as polyene phosphatidylcholine, adenosyl methionine, compound glycyrrhizin, ursodeoxycholic acid, glutathione, antiviral agents (tenofovir or entecavir), antibiotics, diuretics, aspartate ornithine, lactulose, human albumin and fresh plasma.
Combination treatment of DPMAS and low volume PE is the intervention factor in this study. Thus, the 100 participants in the trial group will receive similar treatment of control group, and additional combination treatment of DPMAS and low volume PE for three times with an interval of 2–3 days in the first 2 weeks after enrolment. DPMAS treatment is performed by application of filtration technique with a membrane plasmapheresis apparatus with plasma separator, adsorption products from Jafron Biomedical (HA330-Ⅱ Disposable Hemoperfusion Cartridge and BS330 Disposable Plasma Bilirubin Adsorption Column), extracorporeal blood circuit and dual lumen dialysis catheter according to manufacturer’s protocol. The volume of plasma adsorption in DPMAS treatment is 5000–6000 mL, with a blood flow of 100–120 mL/min and a plasma adsorption rate of 25–30 mL/min. The duration of DPMAS treatment was about 3.5–4 hours. After DPMAS treatment finishes, sequential low volume PE treatment begins. PE treatment is performed with the same membrane plasmapheresis apparatus, the same set of plasma separator, extracorporeal blood circuit and dual lumen dialysis catheter used in DPMAS previously. The volume of fresh plasma or FFP used in PE treatment is 1000 mL, with a blood flow of 100–120 mL/min and a PE rate of 25–30 mL/min. The duration of PE treatment was about 1 hour.
As DPMAS and PE are two modes of ALSS, there are some adverse events (AEs) related to ALSS during the operation course, such as low blood pressure, allergy, infection, bleeding and thrombosis. If the AEs about low blood pressure and allergy are mild, the operation for DPMAS and PE can go on by symptomatic and supportive treatment, such as slowing down the blood flow speed, fluid expansion and antiallergy, with vital signs monitoring. Otherwise, the operation will be stopped if the AEs are life-threatening. The operation must be stopped when infection, bleeding or thrombosis occurs.
All participants will receive the recommended standard of care. Details and precautions of DPMAS and PE are explained to participants preoperation and postoperation, in order to ensure the completion of treatment.
Evaluation and outcomes
Participants’ symptoms, signs, laboratory test results, AEs, unfavored events (death, liver transplantation and treatment abandonment) are recorded at every visit timepoint, see table 1. Symptoms include fatigue, poor appetite, nausea, vomiting, abdomen distention and jaundice. Signs include consciousness, body temperature and vital signs like blood pressure, heartbeat rate, breath rate. Laboratory test results include blood cells (white blood cell, neutrophils, red blood cell, haemoglobin (HGB), platelets (PLT)), biochemical parameters (aspartate aminotransferase, alanine aminotransferase, albumin (ALB), globulin, TBIL, direct bilirubin, blood urea nitrogen, creatinine), coagulation parameters (prothrombin time (PT), PTA, PT-INR). Model for end-stage liver disease (MELD) scoring system is an important measurement tool for short-term mortality risk in patients with end-stage liver disease.17 It is used to evaluate the severity and prognosis of patients with HBV related ACLF in the study. MELD score was calculated using the following formula: MELD=9.57×loge(creatinine mg/dL)+3.78×loge(TBIL mg/dL)+11.20×loge(PT-INR)+6.43.18 AEs refer to any unwanted symptoms or signs in a clinical investigation participant, and do not necessarily have a causal relationship with the applied intervention. For AEs with low morbidity, corresponding treatments will be recommended. AEs leading to stop treatment should be taken as a treatment failure. If any SAE is encountered, it will be reported to the principal investigator of the trial, the ethics committee and the state supervision agency within 24 hours of its occurrence. All AEs will be coded according to the WHO Adverse Reactions Terminology. At the end of the clinical trial, the intensity and relationship of any AEs with the study intervention will be identified. Unfavoured events include death, liver transplantation and treatment abandonment.
The primary efficacy outcome will be the incidence of unfavored events including death, liver transplantation and treatment abandonment. We set up the secondary efficacy outcome as the biochemical parameters including serum TBIL, ALB, creatinine and PT previously. As serum TBIL, creatinine and PT-INR are the key parameters for MELD scoring system. MELD score reflects not only the levels of the above parameters, but also the weight of them. High MELD score relates to severe disease and poor prognosis. Rather than the biochemical parameters including serum TBIL, ALB, creatinine and PT, using MELD score variation does not change the aims of the study which focus on the improvement of severity of patients with HBV related ACLF after treatment. So we change the secondary efficacy outcome into the MELD score variation accordingly the peer review comments. Clinical safety will be assessed by the analysis of AEs and laboratory tests of HGB and PLT.
Participant timeline
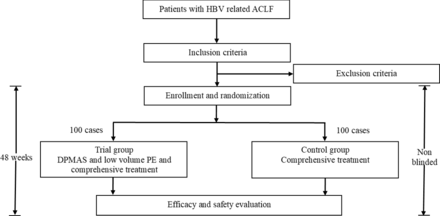
Following baseline assessments, enrolled patients with HBV related ACLF will be randomly allocated into one of the two groups: the control group (comprehensive internal medical treatment, n=100) and the trial group (DPMAS and sequential low volume PE for three times, and comprehensive internal medical treatment, n=100). All evaluations will be performed at baseline, and 4, 8, 12, 24, 36, 48 weeks after enrolment. In trial group, additional evaluations will be performed right before and after the three times of DPMAS and low volume PE treatment (table 1). The schematic diagram is shown in figure 1.
Schematic diagram of combination treatment of DPMAS and low volume PE in patients with HBV related ACLF. ACLF, acute-on-chronic liver failure; DPMAS, double plasma molecular adsorption system; HBV, Hepatitis B virus; PE, plasma exchange.
Sample size
A difference test was used to predict sample sizes in both the trial and control groups. The two-tailed significance level (α) was 0.05. To provide 80% power (1–β) to detect difference in survival rate, β was set as 0.2. After α and β are determined, f(α, β)=7.9. Based on the results of the preliminary clinical trial, the estimated survival rate was 50% in trial group and 30% in control group. Through calculation, N1=N2=90. The maximum possible dropout rate during follow-up was considered to be 10%. Hence, 200 patients will be sufficient to detect differences between the two groups.
Recruitment
All the six trial centres will use the same criteria and rigidity for participants inclusion. Recruitment is done in wards of Department of Infectious Diseases of each centre by the head of centre and his/her medical staff.
Allocation
Randomisation will be performed centrally according to a computer-generated number table which is generated and kept by the research assistant of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The computer-generated number table is unavailable to those who enrol participants or assign interventions. The randomised numbers which are concealed in sealed envelopes will be allocated to research assistants of each centre. The sealed envelopes can only be opened until interventions are assigned to the participants. After assignment to interventions in this study, both those giving therapy and the assessors will not be intervention-blinded, participants will not be treatment-blinded.
Data collection and management
Data collectors, research assistants, assessors, coordinators and investigators will be well trained in advance to be able to competently administer items. Data of participants will be collected immediately at each visit of the planned follow-up if the participants are still in hospital. Reminders of follow-up from coordinators by telephone will be sent to participants if they are discharged from hospital.
Data of participants will be collected and managed with a four-digit pseudonym on a paper case report form (CRF). Six independent trial coordinators will jointly check the completeness and consistency of CRFs in a restricted study office. Then, implausible or missing outcomes will be confirmed or added after consulting the investigator via the data query form and under the supervision of independent trial inspectors. All study documents from the six centres will be considered highly confidential and will be stored in locked filing cabinets in a room with restricted access.
All correct data from paper CRFs that have undergone careful screening will be translated into electronic information and stored in the database. During this procedure, data will be entered twice by two participant-blinded people to allow a double check for accuracy. When data revision is required, the corrected data will be entered by independent trial coordinators under the supervision of both trial investigators and inspectors. Access to the database will be strongly restricted. The principal investigator and biostatistician will be able to log into the data set and get full access to the information only on permission from the head of this study. Data backups and paper CRFs archiving will be performed regularly by trial coordinators. If required, data transfer between centres will be encrypted, and any information capable of identifying individuals will be removed.
Statistical analysis
Continuous data were indicated with mean±SD while categorical data were reported with number and percentage (%). The independent t-test was used to compare means between the two groups. If normality was not assumed, non-parametric tests including Kruskal-Wallis test, Friedman test and Mann-Whitney would be used. Categorical results were compared by χ2 test or Fisher’s exact test (if expected value <5 was found). Kaplan-Meier survival analysis was used to observe the univariate trend of group factor to outcomes. The statistical significance level for all the tests was set at a p value <0.05. Statistical analyses were performed using IBM SPSS V.20 (SPSS Statistics).
Kaplan-Meier survival analysis was used to compare the primary efficacy outcome indicator (the incidence of unfavored events including death, liver transplantation and treatment abandonment) between the two groups. The independent t-test or non-parametric tests will be used to compare the secondary efficacy outcome indicators (serum TBIL, ALB, creatinine and PT) and clinical safety indicators (HGB, PLT) between the two groups. χ2 test or Fisher’s exact test will be used to compare the secondary efficacy outcome indicator (AEs). If required, statistical analysis between subgroups will be performed.
Data monitoring
The final database will be duplicated and uploaded to data monitoring committee (DMC) of the Third Affiliated Hospital of Sun Yat-sen University, to ensure the unique and accuracy of the data. DMC is independent from the sponsor and competing interests.
Termination of the trial can be decided from ethics committee or national administration agency if the clinical trial is proven to be harmful to participants.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Ethics and dissemination
Ethics approval
This study was approved by Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University, China (approval no. (2020)02-173-01) on 29 September 2020 (online supplemental file 2). An informed consent form (online supplemental file 3) that has been approved from the ethics committee will be obtained from all participants before their inclusion. This clinical trial complies with the principles of the World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects, with all its amendments, as well as with the principles of Good Clinical Practice.
Supplemental material
Supplemental material
Informed consent forms and privacy protection
Informed consent forms and associated explanatory information will be clearly explained to participants or their legal representatives by investigators before obtaining their informed consent. Informed consent forms will be prepared in simple language. If trial participants are not able to sign their names, an allograph by their legal representatives will be allowed. No study procedure will be implemented prior to the acquisition of informed consent forms.
Only the investigators and inspectors will have partial access to the personal information of participants during the study procedures. This will occur under the strict supervision of the study’s principal investigator. No investigators will reveal any data associated with this clinical trial, except on an official request by the national administration agency.
If there are any changes to the informed consent form during this clinical study, the revised informed consent form must receive another written approval from the ethics committee before its use, and participants must re-consent to the revised version of the informed consent form.
Additional consent provisions for collection and use of participant data and biological specimens in ancillary studies have already stated in informed consent forms.
Dissemination
The results and conclusions of this clinical trial will be submitted to the chief investigators in the sponsor and participating hospitals, and will be reserved in paper form for at least 30 years. They will be published at academic conferences or in journals. If the results and conclusions are not desirable, although the trial is performed in strict accordance with all standard operating procedures, a paper describing this trial will be written by professional writers in the sponsor hospital. Permission will be obtained in advance from the principal investigator in the sponsor hospital, if investigators in the participating hospitals plan to publish partial results and conclusions of this clinical trial at academic conferences or in journals. When preparing the associated articles, the confidentiality of all subjects will be ensured.
Ethics statements
Patient consent for publication
Acknowledgments
We thank Jafron Biomedical for DPMAS treatment technical support.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
WX, YL and LW contributed equally.
Contributors WX, YL, LW, HG, JC, JY, YO, YG, JL, XL and LP contributed to the concept, design, patient recruitment and follow-up. LP and WX had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. WX, YL and LW contributed to acquisition, analysis and interpretation of data. WX drafted the report. LP obtained funding and took responsibility for the supervision. All authors read and approved the final report.
Funding This study was supported by grants from the National major science and technology project for the prevention and treatment of AIDS and viral hepatitis (No. 2018ZX10302204-002 -002 to LP), Natural Science Foundation of China (No. 81873572 and 82070611 to LP) and the Five-Year Plan of Third Affiliated Hospital of Sun Yat-sen University (No. K00006 to LP). The initiating sponsor and coordinator of this clinical trial is the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Contact information for the trial should be addressed to Liang Peng, telephone: +8613533978874, e-mail: pliang@mail.sysu.edu.cn and Wenxiong Xu, telephone: +8613760783281, e-mail: xuwenx@mail.sysu.edu.cn. The other five participants are Guangzhou Eighth People’s Hospital (Guangzhou, China), Nanfang Hospital of Southern Medical University (Guangzhou, China), The Third People’s Hospital of Shenzhen (Shenzhen, China), Xiangya Hospital of Central South University (Changsha, China) and The First Affiliated Hospital of Anhui Medical University (Anhui, China).
Disclaimer The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, writing, review, or approval of the report; and decision to submit the report for publication.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.