Article Text
Abstract
Objective(s) There has been a recent increase in awareness of the importance of bone health in children treated by paediatric orthopaedic and sports medicine providers. The purpose of this study was to assess our utilisation of 25 hydroxy vitamin D (25(OH)Vit D) testing in the past 10 years, and to evaluate the level of 25(OH)Vit D sufficiency in various populations of patients seen.
Design This is a single site, retrospective medical record review study.
Setting The study took place at a single large, private, paediatric level 1 trauma teaching hospital in the Northeast USA.
Participants Our internal medical records query system identified all patients who have had 25(OH)Vit D testing in the past 10 years, from 1 January 2009 to 31 December 2018. All patients included were seen on an outpatient basis at our Orthopaedic clinics.
Interventions No interventions for strict research, however, eligible patients have had 25(OH)Vit D testing during their standard of care treatment.
Main outcome measure(s) The varying number of 25(OH)Vit D testing that occurred over the study time period within Orthopaedic groups, and by Vit D levels as sufficient, insufficient and deficient. 25(OH)Vit D sufficiency was ≥30 ng/mL, insufficiency <30 ng/mL and deficiency were <20 ng/mL. Patients were stratified and analysed.
Results Between 2009 and 2018, there were 4426 patients who had 25(OH)Vit D testing. Vitamin D testing increased significantly (p<0.001) in the past 10 years. 43% of patients had sufficient 25(OH)Vit D levels, 41% had insufficient levels and 15% had deficient levels.
Conclusion More frequent testing has led to an increased identification of patients with insufficient and deficient 25(OH)Vit D levels. We found over 50% of patients tested were found to have 25(OH)Vit D levels under 30 ng/mL. There should be an increased awareness of patients with orthopaedic problems who may present with 25(OH) insufficiency.
- sports medicine
- paediatric orthopaedics
- paediatrics
- nutrition & dietetics
- preventive medicine
- primary care
Data availability statement
Data are available upon reasonable request. We are open to sharing our raw data from which we based this study on, upon request. Data are available by emailing SM and NS at susan.mahan@childrens.harvard.edu and nicholas.sullivan@childrens.harvard.edu, respectively.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- sports medicine
- paediatric orthopaedics
- paediatrics
- nutrition & dietetics
- preventive medicine
- primary care
Strengths and limitations of this study
Large longitudinal study in single orthopaedic and sports medicine centre.
Able to assess vitamin D testing and trends over 10-year period.
Retrospective study design.
Most of our patients did not have 25(OH)D testing, and we can only report on those who did.
Cannot assess vitamin D intake or treatment.
Introduction
There is an increasing awareness of the importance of vitamin D for the normal development of the paediatric skeleton and for maintaining musculoskeletal health. Historically, the primary source for vitamin D was from exposure to the sun with limited amounts coming from dietary intake, however with increasing use of sun protection methods to avoid skin cancer, the absorption of vitamin D from the sun is decreasing.1 In the musculoskeletal system, vitamin D maintains the skeletal calcium balance by promoting calcium and phosphorus absorption in the gastrointestinal system, improving calcium retention in the renal system and improving mineralisation and strength to the bone.2,3 Risk factors for vitamin D deficiency include breast feeding without vitamin D supplementation, increased skin pigmentation, obesity, older age, geographic latitude, decreased dietary vitamin D intake and decreased sun exposure.3 4 One study found that the prevalence of vitamin D deficiency (25(OH)D<20 ng/mL) at the end of winter in healthy adults who drink milk and take a daily multivitamin was as high as 32%.5 The rates of 25(OH)D deficiency in adolescents have been reported to range from 17% to 47%.6–8 Vitamin D deficiency has been shown to be a risk factor for severe fractures in children.9
Universal screening for vitamin D is not currently recommended in healthy children. Screening is currently only recommended for children at risk for vitamin D deficiency.10 The appropriate blood test to assess vitamin D status is 25-hydroxyvitamin D (25(OH)D), the storage form of vitamin D; the other available test for 1,25-dihydroxyvitamin D (1,25(OH)D), the active form of vitamin D, which is recommended for patients with dysregulated vitamin D metabolism (eg, renal disease) should not be utilised for assessing vitamin D status in most people.10 The serum 25(OH)D level reflects the amount of vitamin D (cholecalciferol and ergocalciferol) obtained from the diet and vitamin supplementation as well as the generation of cholecalciferol (vitamin D3) in the skin on exposure to ultraviolet B radiation; this also reflects the level of body stores of vitamin D. The Endocrine’s Society’s Clinical Practice Guideline defines vitamin D deficiency as 25(OH)D<20 ng/mL, insufficiency as 21–29 ng/mL and sufficiency as 30–100 ng/mL.10 11 The Institute of Medicine has different levels of 25(OH) which constitute deficiency, but this debate is outside the scope of this paper.11 12
Children with 25(OH)D deficiency often present to the orthopedist with musculoskeletal problems including fractures, bony pain, skeletal deformity such as genu valgum/varum and radiographic changes consistent with rickets.13 There have been inconsistent reports of the impact of 25(OH)D in the orthopaedic literature, however, many studies have found association of low 25(OH)D with increased odds for fractures,14 15 lower limb deformities,13 female athlete triad,16 slipped capital femoral epiphysis17 and recently an increasing recognition of its role in progression and pathogenesis of scoliosis.18 Vitamin D deficiency has also been reported in patients with neuromuscular diseases, including cerebral palsy and may contribute to bone fragility particularly in these non-ambulatory patients.19–21
Providers in our Paediatric Orthopaedic and Sports Medicine department have become increasingly aware of the possibility of 25(OH)D deficiency, its prevalence in our northeastern US location, and the problems associated with musculoskeletal health. The purpose of this study was to assess our utilisation of 25(OH)D testing over the past 10 years and to evaluate the level of 25(OH)D insufficiency and deficiency in various populations of patients seen in our department. In addition, risk factors for insufficiency and deficiency were assessed.
Methods
Cohort selection
After obtaining approval from the hospital’s Institutional Review Board, a retrospective chart review of patients treated in the Orthopaedic and Sport Medicine Centres at a large paediatric academic centre in the northeast was conducted. Data were collected from our hospital based medical records electronic query system from January 2009 to December 2018. Patients were included if they met the following criteria: (1) age 0–20 years, (2) treated in the Orthopaedic or Sports Medicine Centres and (3) had a serum 25(OH)D level collected at the time of the clinic visit. Exclusion criteria: (1) known skeletal dysplasia or metabolic disorders such as osteogenesis imperfecta or hypophosphatemic rickets. Patients were grouped into four broad categories based on diagnosis: (1) cerebral palsy/neuromuscular disorders, (2) sports medicine, (3) spine and (4) general paediatric orthopaedics (not otherwise specified above). Patients were grouped into categories based on the type of physician seen at the time of serum 25(OH)D collection: patients seen by a sports medicine physician were placed into the category of ‘sports’; patients seen by the spine team were placed into ‘spine’; patients seen by our neuromuscular physicians were placed into the category ‘cerebral palsy/neuromuscular disorders’ and all other patients seen by our orthopaedic providers, including our bone health clinic endocrinologists, were placed into ‘general’. Our practice has considerable subspecialisation within paediatric orthopaedics to make this differentiation possible. Due to the nature of our medical records electronic query system, individual patient diagnoses for the visit were not available. A sport medicine provider may see a patient with spondylolysis and collect their 25(OH)D and that patient will be categorised as a ‘sports’ patient; similarly, a spine specialist may see a soccer player with scoliosis, and they will be categorised as ‘spine’.
Additionally, we searched our hospital-based medical records electronic query system for all unique patients (no repeat visits) seen in the Department of Orthopaedics and Sports Medicine each year during this time to serve as a volume control for the 25(OH)D testing.
Data collection and classification
Demographic and laboratory data: limited demographic data were collected from the database including age, sex, ethnicity, body mass index, date of visit, orthopaedic treating provider and serum 25(OH)D levels (ng/mL). All serum 25(OH)D levels were processed at the same laboratory at our large paediatric academic centre using mass spectrometry. While there is some debate over the definition of 25(OH)D sufficiency, insufficiency and deficiency in children,10 22 in this study, we classified sufficiency of 25(OH)D based on the Endocrine Society’s recommendation: deficiency <20 ng/mL, insufficiency 20–<30 ng/mL and sufficiency ≥30 ng/mL.10 We also did an additional analysis of patients with a level <10 ng/mL. Patients were categorised into the seasons in which the 25(OH)D testing was completed as follows: spring (1 March– 31 May), summer (1 June–30 August), fall (1 September–30 November) and winter (1 December–29 February). Body mass index (BMI) percentile was categorised according to the WHO categories for children adjusted for sex and age as: underweight (<5th percentile), healthy weight (between 5th and 85th percentile), overweight (≥85th but <95th percentile) and obese (≥95th percentile). Children under the age of 2 years did not have BMI percentiles calculated and were therefore listed in the ‘unknown’ category which was not included in the multivariable analysis.
Data analysis
Descriptive statistics and graphical displays were used to describe the patient cohort. Associations between the four different 25(OH)D levels and patient characteristics were assessed by Spearman’s rank correlation analysis, Cochran Armitage test for trend or Friedmans test, as appropriate. Univariate logistic regression was used to assess associations between insufficient and sufficient 25(OH)D levels and patient characteristics. Furthermore, the multivariable logistic regression analysis was used to assess associations between patient characteristics and the likelihood of testing insufficient in 25(OH)D or testing sufficient for 25(OH)D. Since multiple comparisons were conducted for each cohort characteristic, p-values were adjusted using a Bonferroni adjustment and only adjusted p-values are reported. Statistical significance was set at p<0.05.
Results
Cohort characteristics
From 2009 to 2018, there were a total of 4426 children age birth to 20 years who had their 25(OH)D levels tested. Initial query populated a list of 4527 unique patients (several patients had vitamin D tested more than once but were only included once in the study); 101 patients excluded because of age over 20 years old. The majority of the cohort was female (n=2689/4426, 61%), white (n=2765/4114, 67%) and non-Hispanic (n=3005/4426, 68%) (table 1). 25(OH)D levels for the cohort ranged from <2 to 151 ng/mL. The lowest value (<2 ng/mL) was in a patient noted to have active rickets, and his level normalised after appropriate supplementation. The highest level (151 ng/mL) was in a premature infant who was on vitamin D supplementation in addition to formula in her G-tube; the levels normalised after the supplementation was discontinued.
Cohort summary (n=4426)
From 2009 to 2018, there was 3.9-fold increase in 25(OH)D testing. In 2009, we tested 171 patients for 25(OH)D, about 0.4% of the orthopaedic volume, compared with 787 patients in 2018, about 1.7% of the orthopaedic volume. There was an increase in the rate of 25(OH)D testing between 2014 and 2015. From 2009 to 2014, the average rate of testing was 0.6% and between 2015 and 2018, the rate of testing significantly increased to an average of 1.7% of orthopaedic patients (p<0.001) (figure 1).
Frequency of 25(OH)Vit D tests reported as the number of tests and the percent 25(OH)Vit D tests per orthopaedic volume per year.
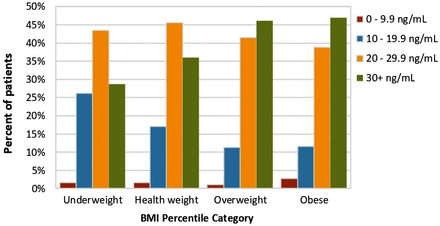
Forty-three percent of the cohort had 25(OH)D levels of at least 30 ng/mL (1925/4426), 41% had 25(OH)D levels between 20 and 29.9 ng/mL (1836/4426), 14% had 25(OH) D levels between 10 and 19.9 ng/mL (600/4426) and 1% had 25(OH)D levels below 10 ng/mL (65/4426) (table 2). There was an inverse relationship present between age and 25(OH)D level group (p=0.006) and varying distribution of 25(OH)D level groups were across increasing age groups (figure 2). There were also differences detected across BMI percentile categories and 25(OH)D levels (p<0.001) (figure 3). Similar trends in the distribution of 25(OH)D levels were seen between spring and winter (colder seasons) and similar trends were seen in summer and fall (warmer seasons) (figure 4). While no significant difference was detected across the seasons, the distribution of 25(OH)D was different when comparing cold to warm seasons (p<0.001). Similarly, while there was no significant difference detected across physician groups, it was found that spine physicians had disproportional higher numbers of patients with low 25(OH)D levels as compared with the other physicians (p<0.001); figure 5.
Cohort characteristics by 25(OH)Vit D level group
Distribution of 25(OH)Vit D laboratory values by age for the cohort (n=4426).
Distribution of 25(OH)Vit D groups by BMI category for the cohort (n=4426). BMI, body mass index.
Distribution of 25(OH)Vit D groups by season for the cohort (n=4426).
Distribution of 25(OH)Vit D groups by physician category for the cohort (n=4426). CP, cerebral palsy; LE, lower extremity.
Table 2 Demographic and clinical characteristics by 25(OH)Vitamin D groups (n=4426)
Multivariable analysis revealed that patients who were older (p<0.001), non-white (p<0.001), obese (p<0.001), visited a spine physicians (p<0.001) or whose level was measured during the colder seasons (p<0.001) had an increased likelihood of 25(OH)D deficiency (<20 mg/mL). Moreover, it was found that for each additional year of age, patients had a 7% increase in the odds of testing 25(OH)D insufficient (OR=1.07 95%, CI=1.04 to 0.1.10, p<0.001). Patients who were obese had 2.4 times greater odds of testing insufficient for 25(OH)D (OR=2.37, 95% CI=1.74 to 3.23, p<0.001). Patients tested in the colder months (winter or spring) had 2.1 times greater odds of testing insufficient for 25(OH)D (OR=2.08, 95% CI=1.62 to 2.66, p<0.001). Patients seen by spine physicians had 1.6 times greater odds of testing insufficient for 25(OH)D (OR=1.64, 95% CI=1.26 to 2.13, p<0.001). Non-white patients had 3.2 times greater odds of testing insufficient for 25(OH)D (OR=3.25 95% CI=2.44 to 4.33, p<0.001).
Discussion
This study demonstrates a steady increase in the number of 25(OH)D measurements obtained for children treated for musculoskeletal disorders in our institution. In those patients tested through our Department of Orthopaedics and Sports Medicine, we found only 43% of patients with sufficient levels of serum 25(OH)D; a surprising 41% were insufficient, and 15% were found to be deficient. Other research has shown variable levels of 25(OH)D insufficiency and deficiency, ranging from 40% to 90%23–25 and our findings are consistent with this wide range. Davies et al22 found that 32% (n=60) of the patients presenting to a paediatric orthopaedic clinic in the UK over a 3year period had 25(OH)D insufficiency, while 8% (n=15) had a deficiency.22 In a retrospective review of all patients seen in a different orthopaedic clinic in the UK in 2010, investigators found that 88% (n=103) of patients had below normal 25(OH)D levels (<30 ng/mL). These low levels were often found in children presenting with limb or back pain.26 Szalay et al23 reported a 40% rate of 25(OH)D deficiency in the orthopaedic patients presenting to their clinic in the American Southwest where more sun exposure typically would be expected.23 Parry et al24 evaluated the serum 25(OH)D levels of 70 patients prior to elective orthopaedic procedures in Texas and found 90% were deficient or insufficient. We also found a higher rate of 25(OH)D deficiency in patients of non-white race, Hispanic ethnicity, presence of obesity, those tested by spine physicians and those in the colder months. It is still not clear what level of low 25(OH)D leads to orthopaedic problems, and this study was not able to determine this; different orthopaedic problems likely have different thresholds where low serum 25(OH)D levels become contributory.
The association of 25(OH)D deficiency with idiopathic scoliosis has recently been shown.18 Several studies have shown that increased scoliosis curve is associated with low serum 25(OH)D levels.27 28 The mechanism of the influence of 25(OH)D on scoliosis is not known and may be multifactorial.18 27 In our study, patients whose 25(OH)D was tested by a spine specialist had a higher rate of deficiency. This is not surprising given the negative correlation seen between 25(OH)D levels and curve progression. 25(OH)D testing is routine in our institution prior to surgery for idiopathic scoliosis.
Increased risk for 25(OH)D deficiency in darker skinned individuals (‘non-white’), particularly for the African-American population, has also been previously reported in other studies.4 24 It is not clear whether or not the 25(OH)D deficiency in African-Americans is associated with decreased bone mineral density and increased risk for fractures in this population.14 In our study, we also found a higher rate of 25(OH)D deficiency among patients of non-white race and Hispanic ethnicity.
25(OH)D deficiency in overweight or obese children has been demonstrated in several studies.6 29 This likely has multifactorial influences including decreased 25(OH)D intake29 as well as possible sequestration of vitamin D in adipose tissue causing the blood level of 25(OH)D to under-represent the total body levels.6 In our study, we also found a higher rate of 25(OH)D deficiency among obese patients.
Seasonal variation in serum 25(OH)D has been shown in many studies, and 25(OH)D levels are lower in the winter months.6 24 This includes studies done in locations such as the southern USA where seasonal variation may be considered less of a factor.6 24 This is likely due to the decreased sun exposure during these months. We also found a higher rate of 25(OH)D deficiency in patients in the winter and spring months.
Currently, there are no recommendations for routine screening or routine supplementation of vitamin D for children and adolescents, although there is increasing recognition that this should be strongly considered.10 25 30 Should we recommend routine screening or supplementation of all children in Massachusetts, the northeast USA, northern climates or all children in general?31 Minkowitz et al recommend 25(OH)D levels be checked on all patients who sustain a fracture to increase compliance of supplementation. However, perhaps all patients should receive supplementation, and that may obviate the need for screening?30,32 While many paediatricians are aware of the problem of vitamin D deficiency, few recommend routine supplementation.30,33 The cost of testing on a population basis can be high34 and this would support more routine supplementation; however, routine adherence to a vitamin D supplementation regime has been difficult for many families.35 There have also been recent endorsements from the American Academy of Paediatrics for higher vitamin D supplementation at higher doses34 instead routine testing for vitamin D deficiency.
Our study had several limitations. Most of our patients did not have 25(OH)D testing, and we can only report on those who did; these patients were clearly selected by their treating physician to be at some risk for 25(OH)D deficiency and some physicians were more likely to test to assess for this possible deficiency. We cannot stratify by diagnosis because the data system we use cannot isolate the diagnosis associated with that visit (most patients have multiple diagnoses). We also cannot assess treatment because, due to a 3-day delay in the 25(OH)D laboratory testing, patients were contacted after their visit and often a multivitamin or over-the-counter vitamin D was prescribed; this is often not reflected in the medical record. We also do not know if the children tested were taking vitamin D supplement (or a multivitamin containing vitamin D) prior to testing, using sunscreen or obtaining significant vitamin D in their diet. Finally, we could not identify patients who had conditions affecting vitamin D intake, absorption and metabolism or children taking drugs that interfere with vitamin D metabolism; this relatively small cohort of patients was therefore included in this study and would affect the analyses.
Conclusion
It is important to recognise that children in the northeast USA are ‘at risk’ for low vitamin D and may present with orthopaedic problems along with their insufficiency or deficiency. We as providers need to recognise the risks of 25(OH)D deficiency in this population and the need for either increased supplementation or screening of 25(OH)D levels in this group.
Data availability statement
Data are available upon reasonable request. We are open to sharing our raw data from which we based this study on, upon request. Data are available by emailing SM and NS at susan.mahan@childrens.harvard.edu and nicholas.sullivan@childrens.harvard.edu, respectively.
Ethics statements
Patient consent for publication
Ethics approval
This study was originally approved and accepted by the Boston Children’s Hospitals (BCH) Internal Review Board (IRB). The IRB approval number at BCH is IRB-P00013551. We applied and obtained a waiver of consent for this retrospective study as contacting over 4000 patients who may have left our institution for treatment would have significantly impacted our patient cohort and would not be reasonably feasible to contact and consent.
References
Footnotes
Contributors In the writing of this manuscript, Dr SM served as the principal investigator and author, with considerable assistance from Drs KA and RDF. Mr NS served as data organiser and assisted in manuscript preparation. Ms PM and Ms LF analysed the data. Dr MG (spine) and Dr IAH (endocrinology) served as specific experts in the manuscript review process. SM is respondible for the overall content as the guarantor and accepts full responsibility for the work and conduct of the study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.