Article Text
Abstract
Introduction Sweat secretion is controlled by the sympathetic nervous system and is less active during winter than in the summer. Raynaud’s phenomenon is affected by an excessive strain of the sympathetic nerves after exposure to a cold environment, thus reducing the quality of life of patients with collagen disease. Herein, we focus on the eccrine sweat glands that receive both adrenergic and cholinergic innervation. Our hypothesis is that excessive activation of sympathetic nerve in Raynaud’s phenomenon can affect sweating, especially in winter. This study is designed to evaluate the neuroactive sweating responses in patients with collagen disease and to assess its association with skin findings in peripheral circulatory disorders.
Methods and analysis The study will be conducted at a single centre in Japan. Patients with systemic sclerosis, Sjogren’s syndrome, systemic lupus erythematosus, mixed connective tissue disease, and dermatomyositis will be assessed using the quantitative sudomotor axon reflex test. The primary outcomes will be sweat volume and reaction time due to axon reflex and the Raynaud’s condition score. The secondary outcomes will include patient background, skin symptoms (digital ulcers, pernio-like eruptions, subcutaneous calcifications, telangiectasia, nailfold capillary dilatation/bleeding and degree of skin sclerosis) and skin surface temperature. Evaluation will be done two times, during the summer and winter, allowing for the assessment of seasonal differences in sweating responses.
Ethics and dissemination Ethical approval of this study was certified by the clinical research review board of Nagasaki University Hospital (Reference number: CRB19-001). We will disseminate the findings of this study through peer-reviewed publications and conference presentations.
Trial registration number jRCTs072190009; pre-results.
- dermatology
- rheumatology
- qualitative research
- statistics & research methods
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study uses an acetylcholine chloride as an off-label drug, which is widely accepted as a safe drug in the quantitative sudomotor axon reflex test.
This study uses assessment methods that reflect peripheral circulatory disorders in patients with collagen disease, such as Raynaud’s condition score, modified Rodnan total skin thickness score and classification of nailfold capillaries.
This study adopts two evaluation points, summer and winter, to observe effects of temperature on sweating.
The limitation of this study is that the results of axonal reflex sweating do not provide the total amount of sweating and do not indicate direct information on noradrenaline-induced vasoconstriction.
Introduction
The sweat function is known to have varying across seasons,1 and the sweating response is also less active in winter than in summer.2 Eccrine glands receive both cholinergic and adrenergic innervation, and are controlled by the autonomic nervous system.3 Aside from sweating, sympathetic nerve activity also regulates changes in body temperature with respect to the environment via vasoconstrictor nerves primarily associated with norepinephrine. In patients with collagen disease, exposure to cold environments can cause the sympathetic nerves to react abnormally, causing Raynaud’s phenomenon in which small blood vessels contract excessively.4 This causes digital ischaemia and gangrene, especially during winter, thus reducing the quality of life of these patients. Recently, it was reported that subcutaneous injection of botulinum toxin, which was widely used as a treatment for hyperhidrosis,5 was effective for improving Raynaud’s symptoms and digital ulcers.6–8 However, it remains unclear whether its antiperspirant effect contributes to the improvement of Raynaud’s symptoms or digital ulcers in patients with collagen disease. We suspect that peripheral circulatory disorders derived from abnormal sympathetic reactions may have some effects on sweating responses. Therefore, we designed the current trial to evaluate neuroactive sweating in patients with collagen disease and to investigate its association with cutaneous symptoms in peripheral circulation such as Raynaud’s phenomenon.
A previous report described a patient with extensive sclerodermatous skin replacement that developed severe fetal heat stroke, and thus patients with diffuse scleroderma were advised to avoid high ambient temperatures.9 This fact led us to hypothesise that patients with systemic sclerosis (SSc) may have impaired thermoregulation due to their impaired sweating function; they may not be able to adapt to seasonal temperature changes. Histopathological abnormalities of the dermal appendages of patients with SSc were atrophied by broadened collagen bundles in the dermis and changes in peripheral blood vessels.9 Furthermore, abnormalities of eccrine glands have been reported among patients with collagen disease. In particular, epithelial–mesenchymal transition and lymphocyte infiltration have been observed in the eccrine glands of patients with SSc, Sjogren’s syndrome (SS) and systemic lupus erythematosus (SLE),10–12 suggesting a possible impairment of the sweating function. Aside from the reports of atopic dermatitis with SS, however, only a few reports have studied the sweating function in patients with collagen disease.10 13 14
The quantitative sudomotor axon reflex test (QSART) is one of the sympathetic nerve tests using acetylcholine,15 and abnormal values can be manifestations of disorders of postganglionic fibres or of the eccrine sweat glands themselves.16 One feature of this design is to observe the seasonal differences of sweating response by making evaluations during summer and winter. This will be the first study to provide a basis for understanding the sweating function of patients with collagen disease and to contribute to the development of treatment strategies for patients with peripheral circulatory disorders.
Methods and analysis
Study design
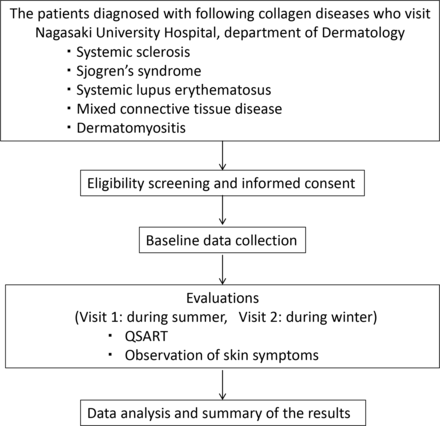
This is an investigator-initiated, single-centre, prospective observational clinical study. The study design is in accordance with the Standard Protocol Items: Recommendations for Interventional Trials and Consolidated Standards of Reporting Trials 2010 guidelines.17 18 Herein, we describe the final protocol (V.2.0; 23 April 2019) for this study. The flow diagram of the study procedure is illustrated in figure 1.
The flow diagram of the study procedure. QSART, quantitative sudomotor axon reflex test.
Recruitment and sample size
The participants are being recruited at Nagasaki University Hospital. Diseases of patients to be recruited will be SSc, SS, SLE, mixed connective tissue disease (MCTD) and dermatomyositis (DM) that cause Raynaud’s phenomenon. Healthy volunteers will be collected as reference values because standard values have not been clarified in QSART. Since this is an exploratory observational study with minimal invasiveness, we aim to recruit as many outpatients as possible who meet the inclusion criteria. The goal of recruitment will be 100 participants, which is based on the actual number of outpatients with collagen disease in our department.
Inclusion criteria
Participants will be selected based on the following inclusion criteria: (1) patients aged 20 years or older who have a collagen disease, including SSc, SS, SLE MCTD and DM; and (2) patients who obtain a thorough explanation of the contents of the explanatory documents and other matters concerning the clinical study, understand the contents thereof and provide written consent based on their own free will to participate in the trial.
Exclusion criteria
The following patients will be excluded: (1) patients who are incompatible for Ovisort (acetylcholine chloride, Daiichi-Sankyo, Tokyo, Japan), (2) patients who have skin abnormalities (eg, dermatitis, skin ulcers, erosion) in the forearms, (3) patients who have a serious allergy to skin dressing materials, and (4) patients judged as inappropriate for any other reason by the clinical investigator or clinical trial physician.
Intervention
QSART was developed to measure the amount of sweating and the duration of sweating in the lateral axon by iontophoresis of cholinergic agonists into the skin.15 Ovisort is a cholinergic agonist. It has multiple uses, and is usually injected subcutaneously for alopecia areata, subcutaneously or intramuscularly for paralysis of the intestinal tract after anaesthesia and for acute gastric dilatation in the case of gastrointestinal hypofunction, or directly into the coronary arteries in the coronary spasm provocation test. In this study, Ovisort will be used as an off-label drug as it will be penetrated into the superficial dermis of the skin for 5 min by iontophoresis. Participants are not allowed to apply moisturisers or topical steroids to the forearm, the measurement site.
Study procedure
The schedule of this study is shown in figure 2. On the first day of observation, the clinical trial physician will explain the study protocol research to patients who were recruited based on the inclusion and exclusion criteria. Afterwards, their written consent to participate in the study will be obtained. Personal information such as patient background and clinical data will be collected from medical records. Patients will be interviewed regarding their daily Raynaud’s symptoms for the past 2 weeks and these will be scored accordingly by the observer. The observation of cutaneous symptoms will be qualified by several dermatologists. The participants will rest in a thermostatic chamber (room temperature 23°C–26°C, humidity 40%–60%) for at least 30 min before the physiological test. Surface temperatures of their fingers will be recorded using a thermography. For further evaluation purpose, a blood sample from each participant will be collected and maintained at the first visit of the observation period. Safety will be also assessed for systemic and local symptoms after QSART.
The schedule of enrolment and assessments. * Visit 1 and visit 2 can come first. QSART, quantitative sudomotor axon reflex test.
Outcome measures
The primary outcome measures include the following:
Sweat volume and reaction time due to axon reflex.
Raynaud’s condition score.
The secondary outcome measures include the following:
1.Patient background, including gender, age, type and duration of collagen disease, history of digital ulcers, presence of pulmonary hypertension, interstitial pneumonia, or reflux esophagitis, smoking history, occupational history, comorbidities (diabetes, dyslipidaemia, hypertension, etc), history of treatment (limited to treatment of the present diseases), and existing patient data (previously positive disease-specific autoantibodies, cryoglobulins, anticardiolipin antibodies, rheumatoid factor, recent %DLCO (diffuse capacity for carbon monoxide), estimated pulmonary artery pressure and HbA1c levels).
Skin symptoms, such as digital ulcers, pernio-like eruptions, subcutaneous calcifications, telangiectasia, nailfold capillary dilatation/bleeding and degree of skin sclerosis.
Skin surface temperature.
Evaluation methods
The degree of Raynaud’s phenomenon will be quantified using the patient’s Raynaud’s condition score,19 while the degree of skin sclerosis will be assessed using the modified Rodnan total skin thickness score.20 Observation of nailfold capillary vessels will be performed using a dermoscope and pattern classification.21
Safety assessments
This clinical study will evaluate for the following: (1) systemic symptoms such as clinically significant adverse reactions (low blood pressure, cardiogenic shock, severe arrhythmia, myocardial infarction, cardiac arrest and anaphylaxis) and other adverse reactions (nausea, vomiting, excessive salivation, faecal incontinence, intestinal cramps, urticaria, convulsions, lacrimation and urinary incontinence), and (2) local symptoms such as redness due to irritation by tape.
All adverse events during the study will be dealt with in accordance with the procedure approved by the certified clinical research review board in our hospital. Specifically, systemic symptoms (serious adverse effects) or unanticipated adverse effects should be addressed in conjunction with the cardiovascular department or the emergency department in Nagasaki University Hospital as appropriate, while local adverse events (redness due to stimulation of tape, etc) should be treated with appropriate topical agents.
Data collection and management
The physician will fill out the case registration form (CRF), which includes the patient’s background and the results of the interview, inspection, palpation, physiological tests, blood tests and safety assessment. All items on the CRF (except the consent and patient’s background) will be observed and described on two visit days, one in the warm season and one in the cold season. Appropriate and authorised persons (investigators, clinical trial physicians and clinical trial collaborators) will be in charge of preparing the CRF. All data recorded in the CRF must be consistent with the original material unless the data recorded directly in the CRF is used as the source material. According to the schedule presented in figure 2, the investigator will collect data at each visit during the study.
The consent form will be submitted to the research office at the time of acquisition, then the CRF will be submitted to the research office within 7 days. All study findings and documents will be regarded as confidential. The research subjects will be assigned identification codes comprised of numeric symbols that are independent of information that can identify a specific individual. Anonymisation will be achieved by using this identification code in the CRFs. The documents related to this research will be strictly controlled in a lockable storage room, and serum collected from the research subjects will be strictly controlled in a lockable freezer.
Statistical analysis
The relationship between QSART measurements (sweat volume and reaction time due to axon reflex) and clinical evaluation will be determined using the least squares method as the regression coefficient of a linear regression model, wherein the Raynaud’s condition score is the dependent variable and each measurement value of QSART is the explanatory variable. Furthermore, for each QSART value associated with these linear regression equations, a scatter plot will be created with the measured value and the residual as the axis. Using the scatter plot, the relationships between the patient background data, the value of specific autoantibodies and the residual will be evaluated. Aside from being a visual evaluation, clustering using distance on a plot will be also be achieved, and the purity of the cluster will be expressed using Shannon entropy.
Significance levels are not set in the study design because the objectives of this study do not fit the hypothesis-based approach to analysis. The multiplicity of tests will be addressed in a manner consistent with the purpose of the analysis. All analyses will be performed in an R environment, and the analysis source code will be made available to the public for reproducibility of the results.
Patient and public involvement
More than half of the outpatients with collagen disease in our department are patients with SSc, including those with severe Raynaud’s phenomenon and digital ulcers. These events reduce their quality of life, especially during winter, while some patients have no or alleviated such symptoms during summer. They were consulted prior to the trial design, as a result, this study was designed from patient’s feedback to evaluate whether seasonal differences in circulatory disorders are associated with sweating of the same sympathetic innervation. The outcome measures used in this study are considered important endpoints in that they can quantify and compare the extent of Raynaud’s phenomenon. In the future, we will report a brief summary using plain language to all participants when the results are published.
Ethics and dissemination
The study is being conducted in accordance with the principles of the Declaration of Helsinki22 and the Japan Clinical Trials Act. Ethical approval for this study has been obtained from the certified clinical research review board of Nagasaki University Hospital (Reference number: CRB19-001). Any modifications to the protocol will be immediately communicated to all responsible authorities.
Discussion
In this study, Ovisort will be used as an off-label drug. It is commonly applied in QSART.23 This is expected to be safe because the actual amount of drug solution will be smaller than the amount commonly used for direct injection and will be rapidly decomposed by cholinesterase. No major side effects have been reported so far.
Sweat volume and reaction time due to axon reflex and the Raynaud’s condition score are set as the primary outcomes because of the simple and safe assessment of perspiration and quantification of Raynaud’s phenomenon, respectively. The degree of skin sclerosis is included in the secondary outcome, since a previous study in Japan reported an association between harder skin and lower basal sweating in scleroderma patients.24
This study is designed for evaluation at two points, the summer season and the winter season, to observe sweating responses to seasonal temperature changes and seasonal effects on peripheral circulatory disorders. According to the Japan Meteorological Agency, the hottest and coldest months in Nagasaki are August and January, respectively. Therefore, 4 months each centred on August and January are set as the summer and the winter observation periods, respectively, (summer season: 1 June to 30 September; winter season: 1 December to 31 March of the following year).
Limitation and expectation
The limitation of this study is that the results from axon reflex sweating do not provide the total amount of sweating. Moreover, they do not indicate direct information on noradrenaline-induced vasoconstriction. We expect to combine other autonomic nervous system assessments with more detailed patient background to better understanding of signs and biomarkers associated with peripheral nerve disorders in patients diagnosed with collagen disease.
Trial status
The trial started on 1 July 2019 and is currently recruiting.
References
Footnotes
Contributors MA, TK and HM were responsible for conceiving and designing the trial, planning data analysis, drafting the manuscript, making the final decision to terminate the trial and approving the final manuscript. MA, DE, YK and HM were participated in data collection and were in charge of recruitment and treatment of patients. MY performed QSART on the participants. SM was responsible for planning data analysis and analysed the data resulting from the trial. MA and TK contributed equally to this work. All authors had access to the interim results as well as the capacity to discuss, revise and approve the final manuscript.
Funding The study is funded by Mitsubishi Tanabe Pharmaceutical Co., Ltd (Grant no. MTPS20190606019). The funding body provides financial support for beneficiary to perform research and publish articles. Specifically, they cover personnel expenses, equipment expenses, travel expenses and other necessary expenses. The trial is sponsored by Nagasaki University Graduate School of Biomedical Sciences (the sponsor representative is Dr Miwa Ashida; Tel.: +81-95-819-7333. Email: ashida@nagasaki-u.ac.jp).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent The informed consent must be obtained from all participants or their legal representative, including healthy volunteers, before study by the recruiting physicians. They will make sure that they can participate in and withdraw from this study at any time on their own free will. The consent form is written in plain language.
Provenance and peer review Not commissioned; externally peer reviewed.