Article Text
Abstract
Objectives There is a lack of information on cotinine levels in rural populations in low-income and middle-income countries like Guatemala. Therefore, there is a need to explore smoking status and biomarkers of tobacco use in epidemiological research in rural, low-income populations, in particular those at-risk for chronic kidney disease of unknown origin (CKDu).
Design We evaluated self-reported smoking status against urinary cotinine levels, the gold standard biomarker of tobacco smoke exposure, among agricultural workers at four separate cross-sectional time points.
Setting Guatemala.
Participants 283 sugarcane workers.
Primary outcome measures Compared self-reported smoking status and urinary cotinine levels in two agricultural worker studies.
Results Self-reported smoking prevalence was 12% among workers. According to cotinine levels (≥50 ng/mL), the smoking prevalence was 34%. Self-reported smoking status had 28% sensitivity and 96% specificity. Urinary cotinine levels show that smoking prevalence is underestimated in this worker population.
Conclusions According to our findings, smoking status should be objectively measured with biomarkers rather than self-reported in CKDu epidemiological research. Self-reported smoking status is likely an underestimate of the true smoking prevalence among agricultural workers. Research on the CKDu epidemic in Central America and other parts of the world might be underestimating tobacco exposure as a potential contributor to the development of CKDu.
- chronic renal failure
- occupational & industrial medicine
- public health
Data availability statement
Data are available upon reasonable request. Data Sharing Agreement: The data that support the findings of this study are available from the corresponding author, JBD, upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The study provides an international view of the importance of adequately assessing smoking prevalence by validating the accuracy of self-reported smoking questionnaires.
The misclassification bias of smokers needs to be examined in rural populations.
Urine cotinine and self-reported smoking status were investigated concurrently at multiple cross-sectional time points.
The study results may have limited generalisability as it was conducted among agricultural workers in Guatemala.
Introduction
Smoking as a potential contributor to the development of chronic kidney disease of unknown origin (CKDu) has not been fully explored and is often overlooked in CKDu research. The emerging CKDu epidemic has been documented over the past two decades throughout Latin America, Sri Lanka and India.1 CKDu is not associated with established CKD risk factors such as diabetes or hypertension and the aetiology remains unknown.1 Several CKDu risk factors have been proposed, including heat stress, dehydration, environmental exposures, infectious agents, medications, as well as a multifactorial aetiology.1–6
Smoking, as opposed to other CKD risk factors, has received less attention in epidemiological studies in populations at risk for CKDu despite the evidence of its role with CKD. Scientific literature provides both mechanistic and epidemiological evidence linking smoking to kidney disease and it is an established and independent CKD risk factor.7–9 While few studies have found an association between self-reported smoking and CKDu,10–13 there may be multiple reasons for the lack of association findings. First, tobacco use misclassification is common and there is considerable heterogeneity between misclassification rates,14 especially among light or non-daily smokers.15 This misclassification potentially leads to the underestimation of the harmful effects of smoking. Here, we present data on the validity of self-reported smoking status against urinary cotinine levels in a sample of workers at risk for CKDu in Guatemala. Cotinine, the main nicotine metabolite, accumulates in the body as a result of tobacco exposure and can be easily detected in urine, blood and saliva. Urine cotinine is commonly used as an objective measure to distinguish tobacco users and non-users.16 Second, research on how smoking is assessed in rural populations in low-income countries where CKDu is endemic is lacking. Smoking patterns in CKDu endemic areas may be different from those in urban populations and in high-income countries, where smoking is intermittent.17–20 Third, while smoking may not be the main cause of CKDu, it may serve as an effect modifier or accelerate disease progression, as it is a well-established CKD risk factor.7
Therefore, we provide evidence that smoking should be objectively measured using biomarkers, such as cotinine, in CKDu epidemiological studies and should not be assessed in the same manner as in high-income countries, where smoking practices are likely different.
Methods
Study Design
The data for this analysis were derived from two studies among male agricultural workers (≥18 years) employed by a sugarcane agribusiness in Guatemala. One study was conducted during the 2016–2017 harvest among 81 field workers and the other study during the 2017–2018 harvest among 202 field workers. The harvest season lasts 6 months from November through May.
The 2016–2017 participants were randomly recruited within a population of workers in December 2016. The 2017–2018 participants were a similar but separate population of workers and were randomly recruited within four work groups of workers in November 2017. During the 2016–2017 study, we collected survey data and spot urine samples in February 2017. During the 2017–2018 study, we collected survey data and spot urine samples at three time points: November 2017 (4 groups), January 2018 (2 randomly selected groups among the 4 groups) and April 2018 (4 groups). This gave us a total of 283 matched urine and participant surveys with self-reported smoking status.
For the 2016–2017 study, participants were asked at the end of their 8–10 hour work shift in February: ‘how many cigarettes have you smoked since you woke up this morning?’ by a Spanish-speaking interviewer (not employed by the agribusiness). At enrollment in November for the 2017–2018 study, participants were asked ‘do you smoke cigarettes?’. Participants responded that they were either current smokers, former smokers or had never smoked. Former smokers were also asked ‘how old were you when you quit smoking?’. At the other two time points during the 2017–2018 study, January and April, the participants were asked at the end of their 8–10 hour work shift: ‘how many cigarettes have you smoked since you woke up this morning?’.
Urine samples were collected in morning except at the November time point, where 95 samples (47%) were collected in the afternoon. A common practice with urine analytes is to correct for urine creatinine to adjust for dilutional effects. However, studies documenting the usefulness of correcting cotinine levels for urine creatinine are limited and may not be necessary.21 We did not correct for urine creatinine based on the limited correction information and after establishing that our afternoon urine creatinine levels were dilute, and dehydration was not a concern.
Laboratory analysis
Urine cotinine levels were determined using the Calbiotech Cotinine ELISA CO096D (Calbiotech, El Cajon, California, USA) and the limit of detection was 5 ng/mL. A cotinine-verified non-smoker was defined as having urinary cotinine ≤50 ng/mL. This threshold value was used as the cut-off according to the Society for Research on Nicotine and Tobacco and is consistent with being a current smoker.22
Statistical analysis
We compared self-reported smoking status and urine cotinine categories (≤50 ng/mL vs >50 ng/mL) in November 2017. Participants were excluded (n=50) if they had missing survey data or urine samples. Agreement between self-report and cotinine levels was assessed using the McNemar’s test. We calculated sensitivity (cotinine: >50 ng/mL and reported being a current smoker) and specificity (cotinine: ≤50 ng/mL and reported being a non-smoker) using urinary measurements as the gold standard. Similarly, at the three other time points (February 2017, January 2018 and April 2018), cotinine categories were compared with self-reported cigarette use on the study day. All data analyses were performed in SAS V.9.4.
Patient and public involvement
These two studies included a collaborative process that engaged workers and worker representatives in the development, implementation and dissemination plans of the research to enhance its relevance and impact. They were not involved in the data analysis or interpretation of this research.
Results
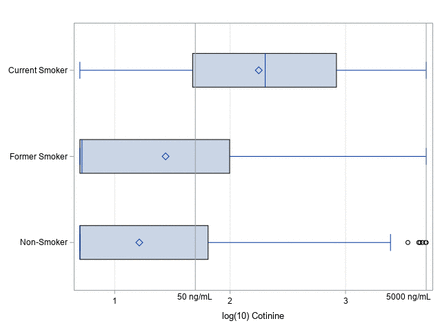
November 2017 urinary cotinine level distributions for the 202 participants are presented in table 1 and figure 1. Among 150 participants (74%) who reported had never smoked, 39 (26%) had cotinine levels >50 ng/mL and among 28 participants (14%) who reported being a former smoker, 10 (36%) had cotinine levels >50 ng/mL. Based on responses to the question, ‘how old were you when you quit smoking?’, none of the former smokers had high cotinine levels due to having just quit. To assess the accuracy of self-reported data, sensitivity and specificity were calculated. Self-reported smoking status had a sensitivity of 28% and specificity of 96%, indicating that 72% of the workers identified as smokers by the urine cotinine test reported being a former or never smoker and 4% of workers identified as non-smokers by the urine cotinine test reported themselves as a current smoker. Smoking status and cotinine levels were not associated (McNemar’s test, p<0.05).
Box plots of log10 urine cotinine levels by self-reported smoking status in November 2017.
Urine cotinine levels by self-reported smoking status in November 2017, N=202
For both the studies, the percent of participants who reported no cigarettes on the day of the study but had cotinine levels >50 ng/mL was 21% in February 2017, 26% in January 2018 and 25% in April 2018 (table 2).
Urinary cotinine levels (≤50 ng/mL vs >50 ng/mL) by reported cigarette use on study day, n (%)
Therefore, using these two study populations of agricultural field workers, we found that approximately 25% of participants who would be considered non-smokers based on self-reported smoking status had an objective measurement of recent tobacco exposure with urine cotinine concentration of >50 ng/mL.
Discussion
In this study, we evaluated self-reported smoking status of agricultural workers using an established biomarker of smoke exposure, urinary cotinine. We observed that self-reported smoking status likely underestimates smoking prevalence in this population. The prevalence of smoking was 34% based on cotinine and 12% based on self-reported data, indicating that self-reporting led to an underestimation of smoking by 64%. The self-reported smoking prevalence in our study was much lower than the last national survey on smoking in Guatemala (2003), where 24% of males self-reported as current smokers (definition: smoked ≥1 cigarettes in the past 30 days).23 24
Data on smoking exposure in CKDu studies have mostly been dependent on self‐reports and yielded conflicting findings on the association with kidney dysfunction. Several studies have found that current or ever smokers are at a higher risk of kidney dysfunction. Among 330 sugarcane workers in Guatemala, we found that self-reported current smokers (vs never or former smokers) were at a significantly greater risk for a decline in kidney function over a harvest season.10 Two studies conducted in Sri Lankan agricultural workers found that smoking (current or ever) was a risk factor for CKDu.12 13 In addition, patients with biopsy-proven tubulointerstitial kidney disease in Sri Lanka were more likely to have ever used tobacco.11 Three studies in Nicaragua have found smoking to be a risk factor in univariate analyses and smoking was either controlled for in the multivariable analysis or was no longer significant.25–27 Other studies found no relationships between smoking and kidney dysfunction and/or very low smoking prevalence .28–32 There was a wide prevalence range of self-reported smoking among these community and worker studies. While smoking assessments varied substantially, the most common question to assess smoking was whether participants were current or ever smokers.
Tobacco use misclassification could introduce bias and be one reason for these conflicting results; true smoking rates are likely underestimated as our current findings yield. This misclassification bias could potentially be leading to the underestimation of the harmful effects of smoking on populations at risk for CKDu. In addition, rates of smoking misclassification have been found to be higher in diseased groups and in case–control studies, suggesting that the presence of disease may affect smoking status response.14
Smoking status misclassification is likely due to several reasons. One is the social desirability bias, where smokers misclassify themselves as non-smokers due to cultural pressure to quit smoking. In addition, these data were collected at a worksite with a smoke-free workplace policy in place. Although workers were assured that their survey responses would be kept confidential, they may have felt pressure to deny smoking. Another reason is the difference in smoking patterns between high-income countries and low-income and middle-income countries (LMICs), where CKDu is endemic. One study found differing patterns of current smoking and type of cigarette smoked (light vs regular) across Brazil, China, Mexico and Poland.33 In Guatemala, like other Latin American countries and Latinos in the USA, light smoking and non-daily smoking are highly prevalent.19 20 Furthermore, single-cigarette sales are very common in Guatemala City and neighbouring towns.17 In another study conducted between 2001 and 2004, it was found that non-daily smoking was common among men in several Central American countries (42% in El Salvador, 23% in Guatemala and 19% in Honduras).19 These studies provide insight on smoking patterns in CKDu endemic countries in Central America; it is very possible that light or non-daily smokers do not consider themselves ‘smokers’ and may under-report their cigarette use in epidemiological surveys.34 35 Capturing different smoking patterns among populations at risk for CKDu is an essential step toward accurately documenting tobacco smoke exposure in epidemiological research. While survey-based, self-reported smoking is commonly used to assess smoking status due to its low cost, and ease of use, investigators should be cautious when interpreting smoking prevalence given our findings that self-reported smoking status can be inaccurate. Regular validity tests (ie, biomarkers of smoke exposure) should be performed to compensate for the limitations of self-reported smoking surveys. In addition, questions should aim to capture patterns of both daily and non-daily smokers. A 2014 Global Adult Tobacco Survey stressed the importance of three basic questions to measure tobacco smoking prevalence, which includes questions on current use (both daily and less than daily responses available), past use for non-daily smokers and past use for current non-smokers. These type of improved survey questions on smoking exposure should be evaluated with objective measure, such as cotinine in epidemiological studies of CKDu.
Our study findings have some limitations. It may be difficult to generalise the results as they are from a rural agricultural worker population. While cotinine is commonly used to discern smokers from non-smokers, there may be an overlap between cotinine levels of non-smokers exposed to high levels of secondhand smoke and light/non-daily smokers. Further research should focus on assessing the optimal cut-off point for validating smoking status among agricultural populations in LMICs. While the discrepancy between urinary cotinine levels and cigarette use on the study day is consistent between February 2017, January 2018 and April 2018, we must interpret these findings with caution. A cotinine level of >50 ng/mL might reflect a current smoker who did not smoke any cigarettes on the study day and could reflect smoking the previous day. In addition, 24-hour urine sample collection would be a more reliable parameter for the assessment of diuresis, although less feasible in epidemiological studies.
In this report, we are not taking the position that smoking is the sole cause of CKDu. However, smoking is a well-established and important modifiable risk factor for several diseases, including CKD.7 We have a disease of ‘unknown’ origin and tobacco use has not been fully explored, in part due to self-report misclassification. Thus, understanding unique aspects of smoking is needed among populations at risk for CKDu and future studies need more objective measurements of smoking as a risk factor for the development of CKDu.
Data availability statement
Data are available upon reasonable request. Data Sharing Agreement: The data that support the findings of this study are available from the corresponding author, JBD, upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
All participants provided a written consent prior to enrollment. Both protocols were approved by the institutional review board of the University of Colorado (COMIRB, #16–1824 and #17–1328) and in Guatemala by the Comité de Ética, Facultad de Medicina, Universidad Francisco Marroquin-Hospital Universitario Esperanza (2016–2017 study) and the Comité de Ética Independiente ZUGUEME (2017–2018 study).
Acknowledgments
We wish to thank all our collaborators, including Alex Cruz, Daniel Pilloni and all the workers who have made this work possible.
References
Footnotes
Contributors Conceptualisation: JB-D and JB. Data curation: LK. Formal analysis: WF and JB-D. Funding acquisition: LSN. Methodology: JB-D, SB, LK and LSN. Guarantor: JB-D. Project administration: LK. Visualisation: JB-D, JB and LSN. Writing—original draft: JB-D and SB. Writing—review and editing: JB, LK, SB, WF, CA and LSN.
Funding This study was supported by Centers for Disease Control and Prevention (U19 OH011227) and National Institutes of Health (R21 ES028826), and in part by Pantaleon and by the Chancellor, University of Colorado, CU Anschutz Campus. Funders had no role in data analysis, interpretation of data, writing the manuscript or the decision to submit the findings for publication.
Competing interests University of Colorado and Pantaleon are separate, independent organisations. University of Colorado employed appropriate research methods in keeping with academic freedom, based conclusions on critical analysis of the evidence and reported findings fully and objectively. The terms of this arrangement have been reviewed and approved by the University of Colorado in accordance with its conflict of interest policies.
Provenance and peer review Not commissioned; externally peer reviewed.