Article Text
Abstract
Objectives It can be challenging to manage patients who are anxious during dental procedures. There is a lack of evidence regarding the effectiveness and safety of oral sedation in adults. This study evaluated the effectiveness and safety of oral sedation in patients undergoing dental procedures.
Design Systematic review.
Methods Randomised clinical trials (RCTs) compared the oral use of benzodiazepines and other medications with a placebo or other oral agents in adult patients. A search of the Cochrane (CENTRAL), MEDLINE (via Ovid), EMBASE (via Ovid) and Cumulative Index to Nursing and Allied Health Literature (via Ovid) databases was conducted, without any restrictions on language or date of publication. The primary outcomes included the adverse effects and anxiety level. The secondary outcomes included sedation, satisfaction with the treatment, heart rate, respiratory rate, blood pressure and oxygen saturation. Reviewers, independently and in pairs, assessed each citation for eligibility, performed the data extraction and assessed the risk of bias. A narrative synthesis of the data was provided.
Results A number of RCTs (n=327 patients) assessed the use of benzodiazepines (n=9) and herbal medicines (n=3). We found good satisfaction with treatment after the use of midazolam 7.5 mg or clonidine 150 µg and reduced anxiety with alprazolam (0.5 and 0.75 mg). Midazolam 15 mg promoted greater anxiety reduction than Passiflora incarnata L. 260 mg, while Valeriana officinalis 100 mg and Erythrina mulungu 500 mg were more effective than a placebo. More patients reported adverse effects with midazolam 15 mg. Diazepam 15 mg and V. officinalis 100 mg promoted less change in the heart rate and blood pressure than a placebo.
Conclusions Given the limitations of the findings due to the quality of the included studies and the different comparisons made between interventions, further RCTs are required to confirm the effectiveness and safety of oral sedation in dentistry.
PROSPERO registration number CRD42017057142.
- clinical pharmacology
- health & safety
- public health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
We performed a comprehensive systematic review to identify randomised clinical trials that evaluated the effectiveness and safety of oral sedation in patients undergoing dental surgical procedures.
Anxiety can lead to dental treatment avoidance with consequent exacerbation of poor oral health in phobic patients; therefore, it is important to understand which drugs are effective for anxiety control, as this can contribute to patient compliance to dental treatment.
Adverse effects from oral sedatives are negative outcomes in dentistry that should be avoided; therefore, it is important that we estimate the risk of such effects so that decision-making regarding conscious sedation can be better informed.
This study was carried out with methodological rigour, including explicit eligibility criteria, a broad extensive database search, study selection by reviewers working in pairs and independently and evaluation of the risk of bias.
The quality of the included studies and different comparisons made between interventions were limiting factors for the study findings.
Introduction
Anxiety during dental treatment can cause stress and discomfort in patients and lead to dental treatment avoidance with consequent damage to the oral health of phobic patients.1 2 In this context, effective control of anxiety plays a pivotal role in patient compliance to dental treatment. The use of conscious sedation is an important strategy for the behavioural management of patients who suffer from anxiety over dental treatment.3 Conscious sedation is an approach that uses one or more drugs to produce a state of central nervous system depression while maintaining verbal contact with the patient throughout the procedure.4 The sedation level should be such that the patient remains conscious and can readily understand and respond to verbal instructions or tactile stimulation.5
Indications for the use of conscious sedation include a diagnosis of anxiety and dental phobia, prolonged or traumatic dental procedures and medical conditions potentially aggravated by stress, which can reduce the patient’s ability to cooperate.6
Additionally, the release of endogenous catecholamines can increase the cardiovascular system load in patients with a history of angina, whereas asthmatic patients can present stress-induced acute episodes of breathing difficulty induced by stress. These are among some of the patients’ profiles that can benefit from conscious sedation in reducing exacerbation risk. The risk–benefit should be determined according to the severity of the patient’s condition.7
Oral sedation is one of the relatively accessible means for dental professionals to control patient anxiety. However, oral sedation can have inherent limitations due to the pharmacokinetics of the orally administered drug, such as delayed and variable onsets of action.8 Moreover, drug interventions to provide conscious sedation should have a sufficient safety margin to preclude consciousness loss.9
Benzodiazepines are widely used in oral sedation to induce a state of anxiety in dental procedures.10 These drugs are among the most commonly prescribed and employed for this purpose worldwide.5 8 11 12
Although benzodiazepines have a similar mechanism of action, their pharmacokinetics differ, which are key factors in selecting the best option to suit the patient.13 The different oral sedation options in dentistry include midazolam, diazepam and lorazepam as mainstream drugs, although alprazolam, temazepam and oxazepam have also been used.8
Few studies have synthesised the available evidence on the effectiveness and safety of oral sedation in adults undergoing dental procedures. A systematic review evaluated the safety of using drugs for sedation administered by oral, intranasal, sublingual, intramuscular and intravenous routes in adults undergoing dental procedures. However, the data extraction was not performed in pairs and independently, and the risk of bias or quality of the evidence was not assessed.10 Another systematic review investigated the use of midazolam in dental surgical procedures. Of the ten studies included in the review, only three addressed oral use, while the other studies combined drugs administered orally and via other routes.14
The hypothesis of this study was that conscious oral sedation is effective and safe for use in dental procedures. The gap in knowledge on the use of drugs for oral sedation in dentistry prompted this systematic review to determine the effectiveness and safety of oral sedation drugs in adult patients undergoing dental surgical procedures.
Methods
Protocol registration
The protocol of this systematic review was registered on the PROSPERO—International Prospective Register of Systematic Reviews and also published.15
The population, intervention, comparator and outcomes strategy used was as follows: population, adults requiring dental surgical procedures; intervention, oral sedation; comparator, placebo group or other oral drug administered; and outcomes, effectiveness: anxiety, sedation and satisfaction with the treatment and safety: adverse effect, heart rate, respiratory rate, blood pressure and oxygen saturation.
Patient and public involvement
No patients were involved.
Eligibility criteria of the studies
Inclusion criteria
Participants
Adults requiring dental surgical procedures, such as dental extraction, surgery for orthodontic purposes, removal of residual roots and third molars, dental implants and other dental surgical interventions.
Intervention
At least one group used oral sedation with benzodiazepines or other drugs (eg, herbal medicines).
Comparator
Placebo group or other drug administered orally.
Study
Randomised clinical trials (RCTs).
Exclusion criteria
Studies involving adults with respiratory diseases, with contraindications to benzodiazepine; pregnant and/or breastfeeding women; and those with a history of allergies were not included. Studies that combined the administration of different drugs for oral sedation were also excluded.
Outcomes assessed
Primary outcomes
Effectiveness was measured by improvement in anxiety by using the Dental Anxiety Scale (DAS), Oral Surgery Confidence Questionnaire (OSCQ) and/or other scales for anxiety symptoms.
Safety was measured by the number of participants that reported side effects, number of adverse effects (or adverse drug reactions) and number of participants that dropped out due to side effects.
Secondary outcomes
Secondary outcomes of effectiveness were sedation and satisfaction with the treatment.
Secondary outcomes of safety were heart rate, respiratory rate, blood pressure and oxygen saturation.
Search method for identifying studies
Electronic database search
The following databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL), which includes Dentistry and Oral Health Group’s Specialized Register; MEDLINE (via Ovid); Excerpta Medica Database (EMBASE) (via Ovid); Cumulative Index to Nursing and Allied Health Literature (via Ovid), Lilacs (Scielo) and the Capes database (https://catalogodeteses.capes.gov.br/catalogo-teses/%23!/), without restrictions on language or publication date, with the search encompassing articles published between inception and 12 March 2020.
Other reference search sources
The reviewers (CCB and JOA) manually analysed the reference list or citations of the articles to retrieve and identify other possible eligible studies. The main authors and/or pharmaceutical companies involved in producing the drugs were contacted for information on additional trials, if necessary.
Search strategy
The search was conducted using Medical Subject Headings terms for each oral surgical procedure (such as oral surgery, dental extraction and dental implant), benzodiazepines (and its synonyms) and terms to search for other drugs. The search strategy for MEDLINE (via Ovid) was adapted for each database (online supplemental appendix A).
Supplemental material
Study records
Data management
After performing the search strategies on each electronic database, the researchers imported the results from each search into an EndNote library. Duplicate entries were identified and removed.
Study eligibility determination
Relevant data from the eligible studies were independently extracted into Microsoft Excel, using a standardised data extraction form. Four reviewers (JOA and CCB, CCG and NKA), working in pairs and independently, selected potentially relevant titles and abstracts and applied the eligibility criteria. Full texts of the potentially eligible articles were obtained. Similarly, the reviewers checked the eligibility of each study. Disagreements were resolved by consensus and, when necessary, arbitrated by a third reviewer (RHLM or LCL).
Data extraction
The same reviewers (JOA and CCB, CCG and NKA), working in pairs and independently, used a standardised and pretested form for data extraction. Subsequently, the reviewers extracted the patient data, methods, interventions and outcomes. We contacted the authors for articles with incomplete methods and results data, if necessary. Disagreements were resolved by consensus and, when necessary, arbitrated by a third reviewer (RHLM or LCL).
Risk of bias
A modified version of the Cochrane collaboration approach for assessing the risk of bias was used.16 17 The same reviewers, again in pairs and independently, evaluated the risk of bias for each clinical trial according to randomisation; allocation concealment; blinding of patients, health professionals and outcome assessors; incomplete outcome data; selective outcome reporting; and major baseline imbalance characterising the sample.
The same reviewers attributed the standard answers ‘definitely yes’, ‘probably yes’, ‘probably no’ and ‘definitely no’ for each domain, with ‘definitely yes’ and ‘probably yes’ denoting a low risk of bias and ‘definitely no’ and ‘probably no’ attributing a high risk of bias.18 Disagreements were resolved by consensus and, when necessary, arbitrated by a third reviewer (RHLM or LCL).
Data synthesis and analysis of the quality of evidence
A narrative synthesis of the findings was carried out. The extracted data were summarised in the tables with the measures (mean, SD and absolute and relative frequency).
Heterogeneity was explained by drug doses (higher vs lower) with greater effect than expected at higher doses and treatment time (longer vs shorter).15 Due to the divergences between the drugs prescribed and the doses used and measured outcomes, a meta-analysis was not performed, and the Grading of Recommendations, Assessment, Development and Evaluation could not be produced.19 20
Results
Search strategy results
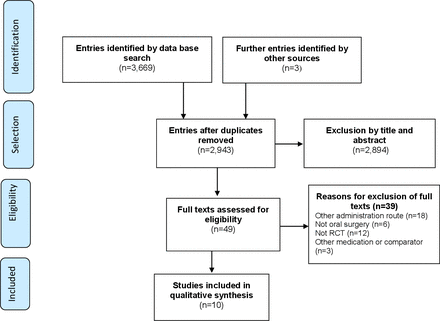
A total of 3,669 publications were retrieved, of which 49 were included for full-text selection. After application of the eligibility criteria, 10 RCTs were included in the review (figure 1). The studies’ characteristics are given in online supplemental appendix B, and the excluded studies are listed in online supplemental appendix C.
Flow chart of study selection process. RCT, randomised clinical trials.
Description of the studies included
The 10 RCTs involved 327 patients (58% women) undergoing oral surgery. Most of the RCTs evaluated the use of benzodiazepines (n=9), and three studies assessed the use of herbal medicines for oral sedation. The majority of the studies were conducted by Brazilian researchers between 2011 and 2017. Only one study was funded by the pharmaceutical industry (table 1).
Characteristics of the studies included (n=10 studies)
Risk of bias
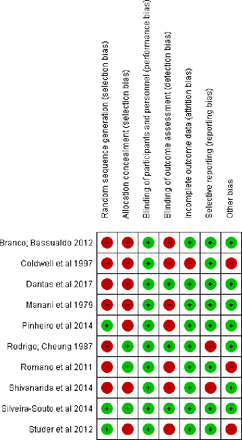
Random sequence generation
Some studies failed to report sufficient data on the randomisation process, precluding any assessment, and exhibited selection bias.21–27 Some stated that patients were randomly allocated to groups but did not detail the process used (figure 2).
Consensus of the authors about bias risk for each study included.
Allocation concealment
Some studies guaranteed that the random sequence generation of participants was unpredictable since the envelopes handed to participants were sealed and coded.22 24 28 By contrast, other clinical trials did not guarantee allocation concealment.21 23 25 27 29 Two clinical trials provided insufficient information on the random sequence generation process employed.26 30
Blinding of the participants and personnel
Rodrigo and Cheung22 and Coldwell et al23 clearly described that the blinding of participants and personnel was ensured and unlikely to have been lost and had no performance bias. The remaining studies stated that they were double-blinded but provided no further details.21 24–30 Consequently, these studies were deemed ‘probably yes’ and considered as having a low risk of bias.
Blinding of the outcome assessors
Blinding of the outcome assessors was performed in three studies, making it unlikely that blinding was lost.22 27 28 Romano et al24 and Pinheiro et al30 stated that the professionals were blinded, but it was unclear whether they were blinded to the outcome collection. The other studies did not report this information, indicating detection bias.21 23 25 26 29
Incomplete outcomes
For the study by Coldwell et al,23 it was impossible to judge whether incomplete outcome reporting occurred. The remaining studies reported whether any participants were lost to follow-up or excluded for another reason.
Selective outcome reporting
One RCT recorded their protocol allowing confirmation that there was no selective outcome reporting.30 Although the study protocol was not reported for the other studies, it appears that they reported all the desired outcomes.
Other sources of bias
Only one study cited the source of funding.22 Other studies declared there was no funding.21 22 25–28 30 The remaining studies did not report sufficient information to assess the presence of other sources of bias.23 24 29
Outcomes assessed
The primary and secondary outcomes reported by the studies are described in tables 2–4.
Primary and secondary outcomes reported by the studies (n=9)
Description of studies that reported the number of participants that experienced adverse effects and dropped out due to adverse effects (n=4)
Description of the adverse effects reported by the included studies (n=5)
Due to differences between drugs used across groups, a meta-analysis of the data could not be performed, and the results were expressed in the form of a narrative synthesis. None of the studies reported sedation outcomes and respiratory rates.
Of the primary outcomes, five studies reported the anxiety levels,23 25 27 28 30 and six studies collected information on the adverse effects.21–23 27 29 30
Table 3 describes the studies that report the percentage of participants who experienced adverse effects. In general, most participants exhibited some adverse effects. Dantas et al27 reported the number of adverse effects but did not report the number of patients with adverse effects. Coldwell et al23 did not specify the adverse effect by group. Therefore, we did not include their results in the table.
The number of reports of adverse effects is shown in table 4. In general, a higher number of adverse effects was associated with the use of midazolam compared with P. incarnata and a placebo, where the most reported adverse effects were drowsiness, muscular relaxation and dizziness.22 27
The secondary outcomes reported were patient satisfaction with treatment (n=1 study),29 heart rate (n=5 studies),21 24 27 28 30 blood pressure (n=5)21 27–30 and oxygen saturation (n=3).26 28
Reporting of the outcomes by drug
Alprazolam (0.25, 0.5 and 0.75 mg)
In a placebo-controlled RCT, 48 participants undergoing dental extraction were allocated into four groups (n=12 per group): group I, alprazolam 0.25 mg; group II, alprazolam 0.50 mg; group III, alprazolam 0.75 mg; and group IV, placebo. Anxiety was assessed using the DAS, OSCQ and the Interval Scale of Anxiety Response . The proportion of individuals that reported feeling fairly to very anxious during oral surgery decreased with increased doses of alprazolam. The most commonly observed adverse effect associated with the use of alprazolam at doses of 0.25, 0.50 and 0.75 mg was anterograde amnesia.23
Diazepam (5, 10 and 15 mg) and lorazepam (1 mg)
In a double-blind and placebo-controlled RCT, 30 participants undergoing dental implant placement surgery were allocated into three groups (n=10 per group). One hour before the procedure, they received the following interventions: group I, diazepam 10 mg; group II, lorazepam 1 mg; and group III, placebo. Anxiety was measured based on Corah’s DAS. No significant difference was found between the groups concerning this outcome.25
An RCT allocated 82 patients undergoing outpatient dental surgery to group I, placebo; group II, trazodone 25 mg; group III, trazodone 50 mg; and group IV, diazepam 15 mg. A comparison of the reported adverse effects for trazodone with diazepam revealed that diazepam was associated with more effects. The main effects reported were drowsiness, vertigo and cognitive impairment. In addition, the number of individuals in use of diazepam reporting adverse effects was also higher. No difference in the heart rate and blood pressure was observed between the groups.21
An RCT with a crossover design (washout not reported) allocated 20 participants undergoing periodontal surgery to group I, diazepam 5 or 10 mg (according to body weight), or group II, placebo, 1 hour before surgery. No significant differences in oxygen saturation were observed between the groups.26
Midazolam (7.5 and 15 mg)
An RCT with a crossover design (washout of 30 days) allocated 12 patients undergoing bilateral surgical third molar extraction to receive the following interventions 1 hour before the procedure: group I, midazolam 7.5 mg, and group II, clonidine 150 µg. The level of satisfaction with the treatment was determined using the Visual Analogue Scale with ratings ranging from ‘no satisfaction’ (0%) to ‘complete satisfaction’ (100%). Around 77% of the patients who received midazolam were satisfied compared with 75% of those given clonidine. There was no difference in the number of participants or adverse effects. No significant difference was observed in heart rate between the groups studied.29
Another RCT allocated 15 participants undergoing implant placement to receive either group I, midazolam 15 mg, or group 2, placebo 1 hour before the procedure. The use of midazolam proved ineffective as a premedication anxiolytic for preventing myocardial arrhythmias.24
An RCT with a crossover design allocated 30 patients undergoing bilateral surgical third molar extraction to group I, midazolam 15 mg (single dose), or group II, placebo 45 min before the dental procedure. They reported a higher number of adverse effects with midazolam compared with placebo, in particular drowsiness, dizziness and excitability.22
Erythrina mulungu 500 mg
The effectiveness of E. mulungu 500 mg (single dose) was assessed in a crossover design RCT (washout period of 15 days) involving 30 patients undergoing bilateral extraction of impacted third molars compared with placebo. Both drugs were administered 1 hour before the dental procedure. Anxiety was determined based on Corah’s DAS Scores. Volunteers with higher anxiety levels tended to prefer herbal medicine. The heart rate, systolic and diastolic blood pressure and oxygen saturation were not significantly different between the groups studied.28
Passiflora incarnata L. 260 mg and midazolam 15 mg
An RCT with a crossover design allocated 40 participants undergoing mandibular third molar extraction into two groups (washout period of 15–30 days). Each group received interventions 30 min before the procedure: group I, 260 mg of P. incarnata and midazolam 15 mg. Corah’s DAS was used before and after the surgical procedure. Both drugs proved to be effective for controlling anxiety, although midazolam 15 mg was more effective than herbal medicine. The most frequent adverse effects, particularly drowsiness and muscular relaxation, occurred with midazolam. The heart rate, systolic and diastolic blood pressure and oxygen saturation were not significantly different between the groups.27
Valeriana officinalis 100 mg
A crossover RCT (washout period of 15 days) allocated 20 participants undergoing bilateral third molar extraction into two groups that received the intervention 1 hour before the procedure: group I, V. officinalis 100 mg, and group II, placebo. Anxiety was measured using the DAS. Herbal medicine was more effective in controlling anxiety than a placebo. No differences were reported in the number of adverse effects, with the most common being drowsiness and muscular relaxation. Herbal medicine promoted less change in the heart rate and blood pressure compared with a placebo.30
Discussion
Main findings and literature comparison
This review has evaluated the available evidence on the effectiveness and safety of oral sedation in adults undergoing dental procedures using 10 RCTs. The majority of the RCTs evaluated benzodiazepine class drugs for oral sedation, where the most commonly used was midazolam. Most of the studies were conducted in Brazil, none of which met all the evaluation criteria for risk of bias. The main methodological flaws were related to randomisation and allocation concealment.
The heterogeneity of the interventions and doses precluded a meta-analysis for all the outcomes assessed being performed. The primary outcomes reported by the studies were anxiety and the adverse effects.21–23 25 27–30
In general, alprazolam (0.5 and 0.75 mg),23 midazolam 15 mg, P. incarnata 260 mg,27 V. officinalis,30 and E. mulungu28 were considered effective for controlling anxiety.
The results revealed a higher number of adverse effects associated with midazolam use,22 27 followed by diazepam.21 In addition, a greater number of patients reported adverse effects from these benzodiazepines. However, these findings should be interpreted with caution, given that the high number of reports might be related to the larger number of participants in these studies. Moreover, these findings are based on reports of only one study, where a lack of comparability between studies hampers any meaningful conclusion.
There was no difference in the number of patients that exhibited adverse effects after using midazolam 7.5 mg and clonidine 150 μg,29 but more adverse effects were reported in the group that received midazolam 15 mg than in the placebo group. These results suggest that an increase in midazolam dose may be associated with a higher number of adverse effects.
No difference was found between midazolam 7.5 mg and clonidine 150 µg regarding satisfaction with the treatment.29 The physiological parameters showed no statistical difference by any intervention. No significant differences in the heart rate or blood pressure were evident when comparing E. mulungu to placebo,28 P. incarnata to midazolam,27 midazolam to clonidine29 and diazepam to placebo or trazodone.21 However, the use of V. officinalis was associated with less change in these parameters relative to a placebo.30 There was no difference in oxygen saturation for the use of E. mulungu versus placebo,28 P. incarnata versus midazolam27 or diazepam versus placebo.26
Although there are public policies aimed at herbal medicines in Brazil, such as the National Program for Medicinal Plants and Herbal Medicines (Decree Number 5813 of 2006), the use of herbal medicines is not common in dentistry. Three RCTs with herbal medicines were from Brazil; this is probably because oral sedation is a common practice in dental procedures compared with other countries that use intravenous and other routes for sedation.
Previous systematic reviews could not be compared with the present study’s findings because the RCTs included in these reviews were not restricted to the oral route.10 14 Also, these reviews failed to report most of the outcomes assessed in the present study.
A previous systematic review assessed the safety of using sedation drugs by any administration route in patients undergoing dental procedures. Ten of the studies included were RCTs, but none of these were included in our study because they used a combination of drugs or other routes of administration. Midazolam was the most commonly used drug, irrespective of the administration route. Although the authors stated that the drug appeared to be safe for sedation, the risk of bias of the studies was not considered, and further clinical trials were suggested to confirm the findings.10
Another systematic review investigated the anxiolytic effect of midazolam in dental surgery, regardless of the administration route.14 Of the ten studies reviewed, three involved oral administration, of which only one RCT was included in the present study since the other clinical trials used a combination of different drugs or alternative routes of administration.29
In the literature, no secondary studies that compared outcomes to the treatment and physiological parameters were found.
Study strengths and limitations
This review was carried out with methodological rigour and evaluated the risk of bias, which has not been performed previously in similar reviews.10 14 The strengths of the present review include its explicit eligibility criteria, broad extensive database search and study selection by reviewers working both independently and in pairs.
The primary studies included are a limiting factor to the findings due to their methodological quality, the non-reporting of clinical outcomes and different comparator groups. This meant that a meta-analysis could not be conducted. Another notable factor was the heterogeneous method of reporting anxiety outcomes among studies.
It is also noteworthy that the vast majority of the RCTs (90%) failed to consider the patient’s anxiety level as a study inclusion criterion. Only one study reported that patients with higher anxiety levels tended to prefer herbal medicine.28 This information is important in that according to the literature, oral sedation can help most patients with mild to moderate levels of fear and anxiety but may be ineffective in patients with high levels of anxiety.11 31
Implications for clinical practice and research
Our findings suggest that benzodiazepines and herbal-based medicines could be safely used for oral sedation in outpatient dental surgical procedures. Dental surgeons should devise surgical plans based on the patient’s condition. This requires a detailed analysis in which the patient’s level of anxiety and fear concerning the procedure is determined so that the most suitable medication can be administered.
None of the RCTs evaluated all of the outcomes proposed to determine the effectiveness and safety of oral sedation in dental surgical procedures. Also, a comparison between the studies was not possible due to the different drugs investigated. Therefore, further clinical trials adopting more methodological rigorous data collection and methodological guidelines should be conducted.
It is important to point out that although the findings of this review are somewhat limited, benzodiazepines and herbal-based medicines both appear to be safe under the conditions reported in the RCTs included (single dose administered orally).
The present review synthesises the available evidence on the effectiveness and safety of oral sedation in adults undergoing dental surgical procedures. This can help guide the decision-making process in dental practice so as to reduce patient anxiety in clinical procedures.
Conclusion
The results suggest that the use of alprazolam, midazolam, P. incarnate, V. officinalis and E. mulungu is effective and safe in controlling anxiety among adult patients undergoing dental interventions. Midazolam was the most studied drug and was associated with the highest rate of adverse effects. However, given the study’s limitations concerning the number of studies reviewed, different comparisons between the studies and incomplete outcome reporting, further clinical trials should be conducted to confirm the effectiveness and safety of these drugs.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @lulopesbr
Contributors JOA contributed as the principal investigator and led the writing of the manuscript. CCB, LCL and RHLM are the project managers and coinvestigators and contributed to the writing and revision of the manuscript. NKA, CCG and JCR are coinvestigators and contributed to the writing and revision of the manuscript. All authors have read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.