Article Text
Abstract
Objectives Identifying the underlying cause of acute coma is crucial for improving outcomes in this time-sensitive medical emergency. This study aimed to explore the clinical characteristics, incidence, causes and outcomes of acute coma.
Design A nationwide population-based retrospective cohort study.
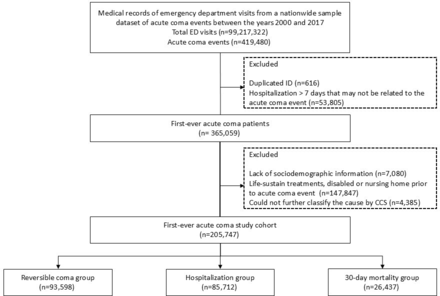
Participants Among 99 217 322 emergency department (ED) visits between 2000 and 2017, 419 480 acute coma events were identified. After excluding visits with only acute coma diagnosis codes lacking detailed information, individuals without socio-demographic data or those with prior nursing home residence or disability, a total of 205 747 first-ever acute coma cases constituted the final research cohort.
Primary and secondary outcome measures The primary outcomes included the acute coma event rate, incidence rates stratified by age and underlying causes categorised into 23 clinical groups by the Agency for Healthcare Research and Quality Clinical Classification Software (CCS). Secondary outcomes assessed were reversible coma, hospitalisation rates, 30-day mortality, 1-year medical utilisation and long-term functional outcomes. Cox regression models identified factors influencing long-term mortality.
Results The overall event rate for acute coma was 4.23 per 1000 ED visits, and the incidence rate was 0.93 per 1000 person-years. The median age of cases was 58.27 years (SD 23.04), with a male predominance (58.90%). Infection and central nervous system (CNS)-related causes were most prevalent. Of these cases, 45.49% experienced reversible coma, 41.66% required hospitalisation and the 30-day mortality group accounted for 12.85%. CNS and drug-related causes contributed to increased 30-day mortality, while psychiatric, alcohol, women’s health and perinatal care, and seizure are causes linked to reversible coma. Patients frequently required intensive care (26.54%), life-sustaining treatments (41.09%) or experienced disability (6.57%) within one year. Generalised estimating equations revealed significantly lower odds of reversible coma for CNS (adjusted OR (aOR), 0.68; 95% CI: 0.62 to 0.74; p<0.0001) and drug-related causes (aOR, 0.72; 95% CI: 0.65 to 0.81; p<0.0001), indicating higher mortality risk. Cox regression analysis showed elevated long-term mortality risks associated with drug-related causes (adjusted HR (aHR), 1.30; 95% CI: 1.20 to 1.41; p<0.001), neoplasms (aHR, 1.18; 95% CI: 1.11 to 1.25; p<0.001) and symptoms-related causes (aHR, 1.44; 95% CI: 1.24 to 1.67; p<0.001).
Conclusion Infection and CNS disorders were identified as the most common aetiologies of acute coma, with CNS and drug-related causes significantly associated with increased short-term and long-term mortality. This study demonstrates the efficacy of using CCS groups for aggregating International Classification of Diseases codes in acute coma research, providing critical insights for enhancing clinical management and outcomes.
- Emergency Departments
- EPIDEMIOLOGY
- INTENSIVE & CRITICAL CARE
- Mortality
- NEUROLOGY
- Clinical Decision-Making
Data availability statement
Data may be obtained from a third party and are not publicly available. Taiwan National Health Insurance Research Database, which was provided by the National Health Insurance Administration, and is managed by National Health Research Institutes.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Emergency Departments
- EPIDEMIOLOGY
- INTENSIVE & CRITICAL CARE
- Mortality
- NEUROLOGY
- Clinical Decision-Making
STRENGTHS AND LIMITATIONS OF THIS STUDY
We used the Agency for Healthcare Research and Quality Clinical Classification Software to develop a clinical research model for investigating acute coma and its clinical characteristics.
This is the first nationwide retrospective cohort study to use longitudinal data, offering insights into the clinical progression and mortality risk of first-ever acute coma.
The proposed research model enables international comparative studies of acute coma, advancing evidence-based practice and supporting the development of artificial intelligence algorithms for acute coma management.
The absence of coma scale data to accurately define the first-ever acute coma cohort represents a limitation, potentially affecting the precision of acute coma incidence estimation.
Heterogeneity in the results may arise from variability in the classification of underlying mechanisms and causes of acute coma across differing definitions, data sets and settings.
Introduction
Acute coma is a critical time-sensitive condition with heterogeneous causes that requires urgent attention and has significant impacts on patients and healthcare professionals.1 It is characterised by profound failure of the neurological system responsible for maintaining arousal and awareness, leading to either a reflex response or no response to external stimuli at all.2 Prior studies estimate that 1–5% of patients presenting to the emergency department (ED) have a disturbance in consciousness.3 4 Emergency care researchers often categorise acute coma into three aetiological factors: primary central nervous system (CNS) disease, severe medical conditions that affect the CNS secondarily or functional such as psychogenic disorder.5 6 The clinical course of acute coma has been classified into three main categories: reversible coma, where patients recover quickly after ED management and can be discharged without any functional deficits; mortality group consisting of patients who do not survive their coma event despite medical interventions; and hospitalisation group, which includes patients requiring hospitalisation that may need intensive care or life-sustaining treatments (LSTs), or complicated with long-term disabilities.7 8 A major challenge in studying acute coma is its heterogeneous nature, with multiple possible contributing factors often present in a single patient. Variations in acute coma study results may arise due to differences in definitions, cause classifications and follow-up periods.9 These factors can affect outcomes and complicate direct comparisons between studies, underscoring the need for standardised methodologies.10 Despite the urgent need for a better understanding of the clinical nature of acute coma, there is a lack of large-scale longitudinal studies that can comprehensively address the incidence, causes, clinical course and outcomes of acute coma.
The Agency for Healthcare Research and Quality (AHRQ) has developed the Clinical Classification Software (CCS) to provide a standardised method for classifying diagnosis codes into CCS categories based on clinical characteristics.11 12 The CCS categories employ the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision, Clinical Modification (ICD-10-CM) classification systems to aggregate large numbers of ICD diagnostic codes into 285 clinically meaningful categories, thereby making clinical research more feasible. Our study aims to (1) estimate acute coma incidence, (2) use the CCS to identify acute coma causes and (3) investigate the clinical course and outcomes.
Methods
Study design and setting
In this nationwide population-based retrospective cohort study, we used the Taiwan National Health Insurance Research Database (NHIRD) to examine ED visits between 1 January 2000 and 31 December 2017. The NHIRD, managed by the Ministry of Health and Welfare, offered a comprehensive data set with information on demographics, comorbidities, hospitalisation, functional status and mortality. This study was conducted with the approval of the local ethics board and involved no direct patient interaction. We carried out a retrospective analysis of claims data, ensuring all personal identifiers were encrypted to uphold patient confidentiality.
Acute coma participants’ definition
Given the nature of this study, we used the NHIRD data set to investigate acute coma incidences. However, the NHIRD data set lacks specific indicators, such as the Glasgow Coma Scale (GCS), to accurately represent coma status. Consequently, we relied on the judgement of emergency physicians in diagnosing acute coma instances, especially in cases where there was no explicit diagnosis but an indication of coma in the ED’s diagnoses. We employed DynaMed (2020) Coma ICD codes to define acute coma objectively.13 These codes encompass a range of acute coma conditions, including ‘780.1’ and ‘780.01’ for comatose, ‘780.09’ for other alterations of consciousness, ‘R40.0’ for somnolence, ‘R40.1’ for stupor, ‘R40.2’ for unspecified coma and ‘S06.7’ for intracranial injury-related coma. Therefore, our study population consisted of cases that included any of these codes within the three diagnoses on ED discharge records and remained as the final research cohort (figure 1). The present study implemented several exclusion criteria to ensure precise estimation of the cause, disease progression and clinical outcomes associated with acute coma. First, we omitted cases lacking comprehensive socio-demographic data. Second, we excluded those who were undergoing LSTs or were disabled or residing in a nursing home prior to the first-ever acute coma event. Additionally, cases diagnosed with acute coma in the ED that the CCS could not further classify due to the absence of additional diagnostic information from the ED or inpatient records were excluded from the study. To rule out hospitalisations potentially unrelated to the acute coma events, we excluded samples in which hospitalisation occurred more than seven days after the acute coma index date.
Flow diagram of the study. CCS, Clinical Classification Software; ED, emergency department.
Incidence estimates
We estimated the annual acute coma event rate from 2000 to 2017, with acute coma events as the unit of analysis. The event rate of acute coma is calculated by dividing the number of events by ED visits. In addition, we identified that crude age group-stratified incidence rates were determined per 1000 person-years, with denominators based on the number of insured individuals during the year, taking into account their survival status and the person-years they contributed within that year. Considering insured individuals’ survival status and person-years contributed and reported age-specific incidence rates in paediatric (1–18), adult (19–64) and older adult (65+) groups with corresponding summary statistics.
Clinical course, causes and outcomes assessment
The study explored the clinical course of acute coma using each patient’s first-ever event as the unit of analysis. The index date was set as the date of the first diagnosis of acute coma. ED visits were categorised into reversible coma, hospitalisation and 30-day mortality.14 Individuals who died within 30 days of the acute coma ED index date were classified as the 30-day mortality group. Those requiring hospitalisation within 7 days post-episode but not dying within 30 days constituted the hospitalisation group. Patients diagnosed with acute coma in the ED without needing hospitalisation or facing death were categorised as the reversible coma group.
Using CCS methodology,12 15 16 we categorised ICD codes from death or hospitalisation into 23 acute coma causes (online supplemental table 1), and a statistical analysis plan is available in online supplemental programme. The diagnosis sequence begins with death, hospitalisation and ED diagnosis if no death or hospitalisation occurs. These causes were further classified into three aetiological mechanisms: (1) primary CNS diseases (neurological aetiology), (2) medical conditions affecting the CNS secondarily (medical aetiology) and (3) functional aetiology.5 Neurological aetiology included acute CNS insult, chronic neurodegenerative encephalopathy, paroxysmal seizure disorders and traumatic brain injury. Medical aetiology included alcohol-related coma, drugs and organ system dysfunction. Functional factors included psychogenic disorders, symptoms, syncope and other related causes. Patients were followed for one year to evaluate short-term outcomes (30-day mortality or reversible course) and long-term outcomes (intensive care unit (ICU) admission, LSTs,17 rehabilitation, disability status or nursing home residency).
Supplemental material
Supplemental material
Statistical analysis
We used χ² tests to analyse baseline categorical characteristics and compared continuous variables' mean among coma groups with one-way ANOVA. Generalised estimating equations (GEEs) were used to estimate acute coma’s adjusted OR (aOR), accounting for multiple causes and covariates like sex, age, Charlson Comorbidity Index (CCI), occupation, urbanisation and income. Survival analysis was conducted for reversible and hospitalisation groups, tracking survival probability and calculating time to event (death) or censoring. Cox regression investigated potential causes of death events, with hazard ratios (HRs) identifying factors affecting long-term outcomes. Analyses were performed with SAS software, V.9.4, and a significance level of p<0.05.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Results
Cohort characteristics and clinical course estimate
Among 99 217 322 ED visits between 2000 and 2017, 419 480 acute coma events were identified. Of these, 365 059 patients were discharged or hospitalised within seven days. After excluding 4385 ED visits with only acute coma diagnosis code, lacking further information, and participants lacking socio-demographic data or with prior nursing home or disabled status, 205 747 cases remained in the final research cohort (figure 1). The cohort clinical course classified 93 598 (45.49%) as reversible acute coma group, 85 712 (41.66%) as hospitalisation group and 26 437 (12.85%) as 30-day mortality group. The study population was 54.39% male, with an average age of 58.27 (SD 23.04) years (online supplemental table 2).
Supplemental material
Incidence of acute coma
Table 1 analyses ICD diagnosis codes for acute coma events, revealing (1) a crude event rate of 4.23 per 1000 ED visits, (2) an average overall incidence rate of 0.93 per 1000 person-years and (3) age-specific incidence rates, 0.13 for paediatric, 0.57 for adult and 7.13 for older adult groups. A significant mean decrease in incidence rate in 2016 suggests that age and temporal factors may influence acute coma incidence.
Acute coma event rate and incidence by year and age group
Causes and outcomes of acute coma
Online supplemental table 1 presents leading acute coma causes, including infection (15.10%), CNS (14.61%), digestive (9.67%), cardiovascular (9.41%) and trauma-related (8.65%). Common reversible causes included infection (15.72%), trauma (10.89%), digestive (10.00%), women’s health and perinatal care (9.56%) and CNS (8.74%). Hospitalisation for acute coma frequently resulted from CNS (17.08%), infection (16.34%), cardiovascular (9.51%), digestive (9.30%) and diabetes and insulin (6.45%). Leading causes of death were CNS (27.40%), cardiovascular (12.41%), digestive (9.73%), trauma (9.10%) and infection (8.87%). Medical aetiologies were the primary factor (66.75%), with neurological (27.60%) and functional (5.65%) aetiologies also contributing. Short-term outcomes indicated 45.49% of cases left the ED without sequelae, 12.85% experienced 30-day mortality and 41.66% necessitated hospitalisation within 7 days. Elderly patients had a significantly higher mortality rate of 62.56% compared with 11.56% for younger patients. The 1-year follow-up showed ICU treatment (26.54%), LSTs (41.09%), rehabilitation (14.23%), disability (6.57%) and nursing care (1.88%).
Multivariate analysis of acute coma
The GEE analysis identified covariates significantly associated with increased acute coma mortality, including females, older age, higher CCI scores, low income and rural residence (online supplemental table 3). Compared with other causes, CNS (aOR, 0.68; 95% CI: 0.62 to 0.74; p<0.0001) and drug-related causes (aOR, 0.72; 95% CI: 0.65 to 0.81; p<0.0001) had lower odds of reversible coma compared with 30-day mortality, while psychiatric (aOR, 57.02; 95% CI: 34.11 to 95.33; p<0.0001), alcohol (aOR, 33.8; 95% CI: 21.81 to 52.38; p<0.0001), women’s health and perinatal care (aOR, 11.86; 95% CI: 10.11 to 13.92; p<0.0001), seizures (aOR, 8.32; 95% CI: 6.15 to 11.24; p<0.0001) and musculoskeletal/integumentary causes (aOR, 8.16; 95% CI: 7.04 to 9.47; p<0.0001) had higher odds. Drug causes had lower odds of hospitalisation compared with mortality (aOR, 0.82; 95% CI: 0.73 to 0.91; p=0.0003), while psychiatry (aOR, 48.29; 95% CI: 28.88 to 80.77; p<0.0001), seizure (aOR, 9.01; 95% CI: 6.67 to 12.17; p<0.0001), women’s health and perinatal care (aOR, 5.44; 95% CI: 4.63 to 6.40; p<0.0001) and alcohol (aOR, 5.20; 95% CI: 3.31 to 8.17; p<0.0001) causes increased the odds. Compared with functional aetiology, neurological aetiology had lower odds of reversible coma (aOR, 0.55; 95% CI: 0.51 to 0.59; p<0.0001) and hospitalisation (aOR, 0.70; 95% CI: 0.65 to 0.75; p<0.0001), while medical aetiology had higher odds of reversible coma (aOR, 1.39; 95% CI: 1.30 to 1.49; p<0.0001) and hospitalisation (aOR, 1.16; 95% CI: 1.09 to 1.25; p<0.0001).
Supplemental material
The Kaplan-Meier estimation (online supplemental figure 1) and Cox proportional hazards regression (table 2) revealed increased mortality risk associated with higher CCI score (adjusted HR (aHR), 1.08; 95% CI: 1.07 to 1.09; p<0.001), older age (aHR, 2.17; 95% CI: 2.13 to 2.22; p<0.001), manual labour (aHR, 1.03; 95% CI: 1.02 to 1.04; p<0.001), drug (aHR, 1.30; 95% CI: 1.20 to 1.41; p<0.001), neoplasm (aHR, 1.18; 95% CI: 1.11 to 1.25; p<0.001) and symptoms cause (aHR, 1.44; 95% CI: 1.24 to 1.67; p<0.001). In addition, the average mortality post-acute coma for the reversible group was observed at 7.10 years, while for the hospitalisation group, it occurred at 6.41 years.
Supplemental material
Multivariate Cox regression analysis of factors contributing to all-cause mortality in patients in acute coma
Sensitivity test of acute coma
To assess the robustness of our findings, we focused on the definition of an acute coma cohort, explicitly examining the first-ever episode that led to hospitalisation within either a 7-day or 14-day period. Our analysis revealed no significant differences between these two cohort definitions in terms of clinical course subgroup distribution and cause classification for acute coma (see online supplemental table 4). This suggests that our findings are consistent and reliable across different definitions.
Supplemental material
Discussion
Acute coma frequently represents a common pathway of organ dysfunction from diverse causes, significantly impacting patients’ survival and quality of life and straining healthcare resources. This study aims to explore the incidence density, causes, clinical courses and outcomes of acute coma. Several methodological and result issues warrant discussion.
Methodology discussion
Our 18-year longitudinal retrospective cohort study employs the ICD coding system and the CCS method to address the complexity of acute coma’s causes and aetiologies. This complexity, driven by a wide range of reversible and time-sensitive factors, poses significant challenges in synthesising diverse clinical causes into a unified cohort for claims-based research. Previous studies have often relied on medical record reviews18 or rigorously designed cohort studies,19 lacking a comprehensive and longitudinal perspective. To bridge this research gap, we devised an innovative clinical research model integrating big data analytics with clinical investigation. This approach offers a novel framework for examining the multifaceted clinical scenarios related to acute coma through claims-based data, thereby opening new avenues for neuroscientific research and enhancing emergency medical decision-making systems.
Study design, population and cohort definition
The Taiwan NHIRD, encompassing the entire population and offering comprehensive medical services, facilitated a thorough analysis of acute coma’s clinical nature. Besides, the large cohort of over 200 000 patients offered a robust population representation. Moreover, we defined the cohort based on one impaired consciousness in the ED study, where the average hospitalisation duration was 6.4 days. Therefore, we included cases where the onset of acute coma and subsequent hospitalisation occurred within 7 days as part of the study cohort.20 By excluding patients with prior nursing home residence or disability status, it provides a better understanding of the true incidence and outcomes of first-ever acute coma.
Meanwhile, the lack of clinical coma scale data raises concerns about the accuracy of the methodology, which relies on ICD coding and the CCS method. Our study employed an expanded definition of acute coma, utilising ICD codes to encompass a broad spectrum of consciousness alterations, including somnolence, stupor, unspecified coma and coma associated with intracranial injuries. This comprehensive approach allowed for the inclusion of diverse clinical presentations.21 We used ICD coding methodology covering the qualitative spectrum of ‘decreased consciousness’, including somnolence, stupor, coma and quantitative GCS score ranges.21 We also included the current quantitative approach to coma assessment, coding GCS scores of 13–15 as R40.0 (somnolence), 9–12 as R40.1 (stupor) and ≤8 as R40.2 (coma, unspecified). This approach ensured a thorough representation of acute coma in our research sample.
Definition of acute coma causes
Integrating CCS with the ICD coding system in clinical research potentially offers a holistic and nuanced methodology for categorising complex clinical data into clinically meaningful classes.15 While established frameworks for transforming a myriad of ICD codes into clinically relevant categories that can guide clinical decision-making, inform policy interventions or enable regular monitoring are not yet widespread,12 in our study, we used CCS to condense 285 CCS categories into 23 clinically relevant causes of acute coma, rendering the study practically feasible and enabling the in-depth analysis of acute coma’s multifaceted clinical manifestations. This approach facilitates large-scale, longitudinal, population-based studies in EDs, optimising approaches to address acute coma’s clinical nature.
Results discussion
Understanding the clinical characteristics of acute coma makes it crucial for intensivist clinicians to identify the cause to prevent disability22 and emergency medical policy applications.
Causes, clinical courses and outcomes
Infections, CNS disorders, digestive issues, cardiovascular events and trauma are the leading causes of acute coma. Our research results are consistent with international findings, with infection being the most common cause.18 23 Acute coma causes differ based on geography24 or age.18 For instance, poisoning contributes to approximately one-third of unconsciousness cases in Nordic countries.24 In children, common causes are intoxication, epilepsy, infection and traumatic brain injury.18 CNS and infectious disorders are more common in adults and older adults.18 21 The prominence of digestive causes for acute coma in our cohort may be due to the prevalence of hepatitis and hepatocellular carcinoma in Taiwan.25 To facilitate a broader understanding of public health implications related to the potential aetiologies and mechanisms underlying acute coma, and to enable meaningful comparisons with existing literature, we have classified the aetiologies of acute coma into three major categories: neurological, medical and functional factors.5 6 26 This categorisation approach aids in developing targeted intervention strategies and informs policymaking. Neurological causes account for about one-third of cases, while non-neurological causes comprise the remaining two-thirds.27 Schmidt reported that neurological and medical aetiologies each contributed to about 50% of acute coma cases.5 Functional or psychogenic coma constituted around 5% of cases. It is worth further exploring the causes of coma resulting from functional factors.
The clinical course of acute coma varies due to differing underlying causes or aetiologies.9 18 Over half of the first-ever patients in acute coma required hospitalisation or faced mortality. In contrast, the other nearly half demonstrated reversible outcomes. The short-term in-hospital mortality rate for patients in acute coma is about 5–11%,3 19 20 with longer follow-up reaching 25%.19 Our study found that 27.60% of acute coma cases were attributed to neurological aetiology, and within the mortality group, 38.16% of cases had a neurological cause. This supports prior research indicating that the clinical course is highly dependent on aetiology.18 Syncope and seizures are generally believed to be the most common causes of reversible coma. However, in our study, these two common causes accounted for only 1.33% of cases of overall acute coma. This may support researchers’ definition of coma as a state of prolonged sustained unconsciousness lasting at least 1 hour.28 Our emergency physicians may better understand syncope and seizure, improving diagnostic accuracy.29 A study showed that 20% of patients in acute coma may have already been reversible on admission.19 If these patients are monitored for 2 months after hospitalisation, one-third of them may fully recover consciousness.30 Our study found that approximately 45.49% of patients had reversible coma. The higher proportion of reversible coma in our study may reflect a more lenient coding of coma or the higher quality of emergency medical care by emergency physicians in our study. These results suggest that the outcome of acute coma is highly dependent on the underlying cause and severity of the condition.31 Regarding long-term outcomes, one-quarter of patients with first-ever acute coma necessitated ICU admission, and 40% required LSTs within 1 year. The high percentage of patients in the LSTs group who require long-term care and have a high mortality rate emphasises the need for improved management strategies for patients in acute coma.7
Incidence
Our study found an acute coma event rate of 4.23 visits per 1000 ED visits, consistent with the Schmidt et al ED cohort study.19 However, our results differ from those of another study that reported 0.29–0.40 cases of coma per 1000 ED visits.32 Based on the ICD code approach, studies suggested that acute coma is about 0.93–5% of all ED visits.27 33 Paediatric non-trauma coma studies also have reported incidences ranging from 0.3 to 1.6 per 1000 person-years.18 This disparity in results may be attributed to the differences in research questions, study design, study population or definitions.34
We investigated the incidence rates of acute coma in different age groups and temporal trends. The highest incidence rate of acute coma was observed in the elderly age group, emphasising the significance of this public health concern in the ageing population. However, there is also some variability in the incidence rates over time. We found that the incidence rate stabilised at around 1 per 1000 person-years from 2007 to 2015 and observed a significant mean decrease in the incidence rate in 2016 compared with previous years. Specifically, there was a significant mean decrease from 0.73 per 1000 person-years in 2016 to 0.63 per 1000 person-years in 2017. One possible explanation for reducing acute coma incidence during 2016–2017 is the transition from the ICD-9 to the ICD-10 coding system in 2015. We also found no significant difference in ED visits between 2014 and 2017 (5 904 262 vs 5 945 444, respectively). Thus, the substantial change in acute coma incidence could be an artefact of the ICD coding transition effect.35
Strengths and limitations
This study has several strengths and limitations. Strengths include using nationwide longitudinal data to observe first-ever acute coma patterns, enabling tracking of clinical progression. The average post-acute coma mortality occurring 7 years highlights its importance as a risk factor and common pathway for mortality. Additionally, the study employed AHRQ CCS methodology, facilitating regular monitoring of acute coma clinical information and enabling tailored intervention plans.
The present study has several limitations that need to be acknowledged. First, the absence of a coma scale to accurately define the first-ever acute coma cohort represents a significant limitation. Instead, the study relied on acute coma-related diagnoses coded by emergency physicians in the ED, potentially leading to an underestimation of acute coma incidence and compromising the accuracy of identifying the causes of coma. Additionally, the conversion between ICD-9 and ICD-10 coding systems may introduce inaccuracies in estimating coma-related diagnoses due to potential discrepancies and inconsistencies in classification. Consequently, the reliability of the results may be affected. Furthermore, it is important to recognise that the acute coma diagnosis employed in this study may not fully capture the underlying causes or medical utilisation as multiple contributing pathologies could be involved due to potential multiple underlying pathologies.19 The complexity of coma aetiology and the potential presence of various underlying factors may limit the accuracy of attributing the diagnosis to a single cause. Moreover, a small proportion (about 2%) of patients in acute coma presented in the ED lacked further diagnostic information, which reflects the challenge in diagnosing cases of coma with unknown origins and introduces potential uncertainty and incomplete data in the analysis. A key limitation of this study is the inability to further explore the association between symptom-related causes and long-term mortality due to insufficient data and significant variability in symptom categorisation. Another limitation is the reliance on data limited to the year 2017, preventing examining the potential effects of the COVID-19 pandemic. Incorporating the impact of the pandemic would have enhanced the understanding of the significance of infections and CNS-related causes in estimating acute coma incidence. Finally, it should be noted that this study did not use WHO’s World Standard Population for age-specific rates adjustment, which may limit the generalisability and comparability of the findings with other studies that use standardised rates based on the WHO standard populations. These limitations should be considered when interpreting the study’s results, and future research should address these limitations to enhance the robustness and applicability of the findings.
Conclusion
Acute coma often represents a common pathway of organ dysfunction with diverse causes or aetiologies, significantly impacting mortality and disability. Our study demonstrates the innovative use of ICD codes aggregation to CCS groups in acute coma clinical study, providing valuable insights into its clinical nature. This research model has the potential to facilitate international comparative studies of acute coma characteristics using healthcare databases.
Data availability statement
Data may be obtained from a third party and are not publicly available. Taiwan National Health Insurance Research Database, which was provided by the National Health Insurance Administration, and is managed by National Health Research Institutes.
Ethics statements
Patient consent for publication
Ethics approval
The study involved a retrospective analysis of encrypted unique personal identification data without direct patient involvement. Therefore, no patient consent was necessary for the completion of this study. No patients were involved. This study was a retrospective claim data analysis that included all encrypted unique personal identification. Ethics approval: IRB of Taipei City Hospital, no. TCHIRB-10807003-E.
Acknowledgments
We acknowledge the Taiwan National Health Insurance Research Database, which was provided by the National Health Insurance Administration and is managed by the National Health Research Institutes. We also thank the Center for Public Health, Department of Education and Research, Taipei City Hospital, for providing administrative support.
References
Footnotes
Contributors C-YL took a lead role in conceptualising the study and writing the original draft, was responsible for formal data analysis and also verified the underlying data in the manuscript. MT contributed to the study design, data curation and formal data analysis and was responsible for data collection. J-FL and M-LC ensured accurate data analysis and interpretation and verified the manuscript’s underlying data. C-CL, Y-CL and M-LC supervised the study, validated the results and significantly contributed to reviewing and editing the manuscript. All authors participated in developing the study concept and design, analysing and interpreting data, and preparing the manuscript. All authors approved the final manuscript and agree to be accountable for all aspects of the work, promising to appropriately investigate and resolve any question related to the work’s accuracy or integrity. M-LC acted as the guarantor. This submission involved the use of AI technology to assist with language editing and academic writing enhancement. Specifically, OpenAI’s language model was used to refine grammar, improve clarity and elevate the overall academic tone of the text.
Funding This study received financial support from Taipei City Hospital.
Competing interests C-YL and M-LC are affiliated with Taipei City Hospital. However, the funder had no role in the study design, data collection, analysis, interpretation, writing of the manuscript or decision to submit for publication.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.