Article Text
Abstract
Objective To evaluate changes in the peripapillary retinal microvasculature and retinal nerve fibre layer (RNFL) in diabetic participants with various stages of diabetic retinopathy (DR) using swept-source optical coherence tomographic angiography (OCTA).
Design Community-based, cross-sectional study.
Setting This study was conducted in a tertiary teaching hospital in Guangzhou, China.
Participants A total of 1325 ocular-treatment-naive participants, of whom 1115 had no DR and 210 had DR, were recruited in a community in Guangzhou, China.
Primary and secondary outcome measures A commercially available OCTA device was used to obtain various peripapillary retinal microvascular metrics centred on the optic disc, including vessel density (VD), vessel length density (VLD) and vessel diameter index (VDI). The peripapillary RNFL thickness was automatically obtained using built-in software. Linear regression analyses were used to evaluate the association of the peripapillary OCTA parameters (VD, VLD and VDI), RNFL thickness with various DR stages and average RNFL thickness with peripapillary OCTA parameters.
Results Moderate and severe DR had progressively decreased VD in the peripapillary ring (β = −0.72, 95% CI = −1.31 to −0.14 and −1.79, 95% CI = −2.81 to −0.77, respectively) and other regions (all p<0.05). Similar changes were observed between peripapillary VLD and moderate and severe DR (all p<0.05). Moderate (β = −4.56, 95% CI = −8.97 to −0.15, p=0.043) and severe DR (β = −10.12, 95% CI = −18.29 to −1.95, p=0.015) had significant thinner peripapillary RNFL in the inferior quadrant. VD and VLD were linearly associated with the average RNFL in the peripapillary ring and average peripapillary area (all p<0.05).
Conclusions The peripapillary retinal microvasculature and RNFL were significantly reduced with the progression of DR, which suggests that monitoring differences in peripapillary microvasculature and the RNFL may be a promising approach to detecting DR progression.
- Diabetic retinopathy
- China
- Diagnostic Imaging
- EPIDEMIOLOGIC STUDIES
- Neuro-ophthalmology
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The present study included a large sample of treatment-naive participants with diabetes mellitus and used a standardised study protocol.
The participants in the present study underwent detailed ocular and systemic examinations with a standardised study protocol.
Study limitations include the following: (1) cross-sectional design and (2) the association between the peripheral retinal layers of the optic nerve head (ONH) and diabetic retinopathy could be examined because only 3 mm × 3 mm scanned images of the ONH were included.
Introduction
The rising prevalence of diabetes mellitus (DM) calls for more attention to diabetic retinopathy (DR), which is the most common microvascular complication of DM and a leading cause of irreversible visual impairment in the working-age population worldwide.1–3 Although significant efforts have been made to elucidate the occurrence and development of DR, its exact pathogenesis remains unclear. Traditionally, DR has been considered a microvascular disease, and most clinical DR grading systems were developed on the basis of the clinical signs of vascular damage. Nonetheless, growing evidence has demonstrated that the neuroretinal structure is affected in the earlier stages of DR or even in its preclinical stage before microvascular lesions are present.4 5 Therefore, understanding the associations of the retinal microvasculature and neuroretinal structure with DR can be helpful in the search for potential mechanisms and the implementation of timely interventions.
The peripapillary area is a viable region for the assessment of retinal neurovascular coupling because it has a peculiar radial anatomical conformation where the capillaries are distributed parallel to the nerve fibre layer axons and provide important nourishment to the retinal nerve fibre layer (RNFL).6–8 Traditionally, fundus fluorescein angiography was the standard imaging approach for peripapillary retinal microvascular alterations. However, the invasive feature and unprocurability of imaging small capillaries by layers restrict the widely studied retinal microvascular networks.9
The introduction of optical coherence tomography angiography (OCTA) has enabled researchers to invasively measure and investigate retinal microvasculature parameters and the RNFL.10 11 Many studies have reported a reduction of the macula microvasculature in diabetic patients with or without DR.12 13 However, quantitative data regarding changes in the peripapillary microvasculature and neurodegeneration in diabetic patients are limited and inconclusive.14 15 Moreover, the association between the changes in peripapillary OCTA features and the RNFL during DR development and progression has not been fully elucidated. To date, although research has revealed that peripapillary microvascular parameters were correlated with the RNFL thickness in patients with DM,16 the results of studies on the direct correlation between peripapillary vascular markers and RNFL thinning in patients with DR have been limited and inconclusive.14 15 Elucidating the exact structural changes of the retinal neurovasculature in the peripapillary region can help to better understand the mechanisms underlying the development and progression of DR.
The purpose of the present study is therefore to explore a quantitative analysis of the peripapillary microvascular and neuronal differences in diabetic participants with various DR stages and further investigate the relationship between the peripapillary microvascular index and the RNFL using OCTA in a large cohort of Chinese participants with type 2 DM (T2DM).
Methods
Study population
This cross-sectional study was performed at the Zhongshan Ophthalmic Center, Guangzhou, China. The detailed methodology has been described previously.17
In general, participants aged 35–80 years who had been diagnosed with T2DM without any eye treatments were recruited from the community of Guangzhou between 1 November 2017 and 30 December 2020. Those with retinal disease and a history of ocular surgeries were excluded from the study. Other exclusion criteria included the following: a history of severe systemic diseases, such as malignant tumours, ischaemic heart disease, uncontrolled hypertension, stroke, cancer or kidney disease; cognitive impairments or mental illnesses resulting in patients being unable to complete the questionnaires and all the examinations; a history of ocular trauma; a history of ocular hypertension or glaucoma; other ocular diseases affecting the neural and vascular structures of the eye (uveitis, optic neuropathy, physiological large cups); a spherical equivalent (SE) > −6.0 D or axial length (AL) >26.0 mm; a best-corrected visual acuity (BCVA) of less than 20/200; and poorly qualified OCTA or fundus images.
The study was approved by the Zhongshan University Ethics Review Board (2017KYPJ094) and was conducted in accordance with the tenets of the Helsinki Declaration. Written informed consent was obtained from all the participants.
Patient and public involvement
Patients and the public were not involved in the study design, conduct of the study or plan to disseminate the result of this study to the study participants.
General information and laboratory parameters
Demographic and systemic information, including age, sex, duration of DM and medical history, were obtained using standardised questionnaires by the same interviewer. Height and weight were measured with participants wearing light clothes and no shoes, using standardised processes, and body mass index (BMI) was calculated as weight in kilograms divided by the square of height in centimetres. Systolic blood pressure (SBP) and diastolic blood pressure were measured after participants had sat quietly for at least 15 min. Haemoglobin A1C (HbA1c), total cholesterol, serum creatinine, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol and triglyceride levels were determined using established standard operating procedures in a laboratory at the Zhongshan Ophthalmic Center certified by the Chinese government.
Ocular examinations
All participants underwent full clinical examinations, including slit-lamp biomicroscopy, ophthalmoscopy, intraocular pressure measurement, uncorrected visual acuity and BCVA evaluation, refraction testing, ocular biometry analysis, retinal photography and OCTA imaging. The ocular biometric parameters—including the corneal diameter, corneal curvature, central corneal thickness, lens thickness (LT) and AL—were measured using a Lenstar LS900 (Haag-Streit AG, Koeniz, Switzerland). Non-cycloplegic refraction was measured using an auto kerato-refractometer (KR-8800; Topcon, Tokyo, Japan), and SE was defined as spherical plus half of the cylinder. The standardised seven-field retinal photographs of each eye adhering to the Early Treatment of Diabetic Retinopathy Study protocol were obtained using a digital fundus camera (Canon CR-2, Tokyo, Japan) after pupil dilation with the instillation of 0.5% tropicamide and 0.5% phenylephrine eye drops. The presence and severity of DR were categorised into no retinopathy, mild, moderate and severe DR by two trained ophthalmologists according to the American Academy of Ophthalmology International Clinical Diabetic Retinopathy Disease Severity Scale and were further confirmed by a senior retina specialist if there was any disagreement between the two ophthalmologists. 18Participants who had missing data were excluded from the current study.
Optical coherence tomography and swept-source OCTA imaging
The optical coherence tomography and OCTA images were obtained using a commercial swept-source OCTA instrument (DRI OCT-2 Triton; Topcon, Tokyo, Japan). A 3 mm × 3 mm Angio Disc mode centring the optic nerve head (ONH) was performed. The ONH was divided into a central grid (central circle with diameters of 1.5 mm) and a peripapillary ring (outer circle with diameters of 2.25 mm). The peripapillary ring was further divided into four quadrants: the superior (S), inferior (I), nasal (N) and temporal (T) quadrants. The ONH microvascular system was automatically segmented into the superficial capillary plexus and the deep capillary plexus using built-in software (IMAGEnet6, V.1.22).19 The thickness of the RNFL in each grid was also obtained automatically using the built-in software. Two experienced investigators checked and adjusted each outline manually to confirm its reliability. All OCTA images were obtained by the same well-trained examiner who had no knowledge of the study protocol. The instrument’s built-in eye movement tracker system was used to minimise motion artefacts, and the artefact elimination algorithm was used for projection artefacts. The image quality of each scan was graded automatically using built-in software (IMAGEnet6, V.1.22), with a quality index ranging from 0 to 100.14 Participants with an image quality <60, residual artefact, poor clarity, signal loss, uncorrected image segmentation error or significant eye movements were excluded from the study.
Image analysis
The OCTA images were standardised, cropped and binarised using Fiji (free downloadable software, https://fiji.sc).12 Before measuring the OCTA features, a binary vessel map and skeleton map were extracted from the input images. The vessel density (VD) was defined as the percentage of the area occupied by vessels in the corresponding region. Vessel length density (VLD) represents the ratio of the total length occupied by all the blood vessels in a given area to the total area based on the length and number of vessels, but not the calibre of vessels. The VD and VLD were calculated in the whole image (wi), peripapillary ring (circ) and the entire peripapillary area (average), which was the part that remained after the 0.752 circle of the optic disc area in the whole image were cut. The vessel diameter index (VDI) of the peripapillary area was obtained by processing both binary and skeletonised images to calculate a vessel calibre index. The details of the image analysis method and sample images have been described in our previously published articles.20
Statistical analysis
For those with bilateral DR, the eyes with more severe DR were analysed. In cases where both eyes were at the same stage or only the right eye was available, we used data from the right eye. The Kolmogorov-Smirnov test was used to verify a normal distribution. When normality was confirmed, a Student’s t-test was conducted to evaluate the intergroup difference for the continuous variables, and the χ2 test was used for the categorical variables. Pearson correlation analyses were used to assess the correlation between the OCTA parameters (VD, VLD and VDI) and the RNFL of the subquadrants. Univariable and multivariable linear regression analyses were used to evaluate the associations of the peripapillary OCTA parameters (VD, VLD and VDI) and RNFL thickness with various stages of DR after adjusting for age, sex, duration of diabetes, HbA1c level, body mass index, systolic blood pressure, total cholesterol level, axial length, intraocular pressure and OCTA signal strength intensity. Furthermore, linear regression analyses were performed to assess the associations of average RNFL thickness and peripapillary OCTA parameters (VD, VLD and VDI) after adjusting for age, sex, duration of diabetes, HbA1c, body mass index, systolic blood pressure, total cholesterol, severity of diabetic retinopathy, axial length, intraocular pressure and OCTA signal strength intensity. These confounding factors were selected based on findings from the Guangzhou Diabetic Eye Study and other relevant studies.21–26 All the analyses were performed using Stata V.14.0 (Stata, College Station, TX, USA). Statistical significance was set at p<0.05.
Results
Participants’ basic characteristics
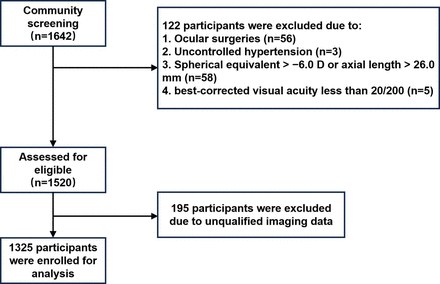
A total of 1325 diabetic participants, of whom 210 (15.8%) had DR, were included in the final analysis (figure 1). Among the 210 participants with DR, 47 (22.4%), 125 (59.5%) and 38 (18.1%) had mild, moderate and severe DR, respectively. The details of the participants’ basic characteristics are presented in table 1. Significant differences in sex (p=0.014), duration of diabetes (p<0.001), HbA1c level (p<0.001), SBP (p=0.001), serum creatinine level (p<0.001), BCVA (p=0.009) and LT (p=0.022) were found between the participants with DR and those without DR.
Flowchart of the included participants.
Demographics and clinical characteristics of study participants
Peripapillary retinal microvasculature and diabetic retinopathy
Compared with the non-DR participants, the DR participants had smaller VD in the whole area, the peripapillary ring, the average peripapillary area and all the subquadrants of the ONH (all p<0.05; table 2). In addition, the VD in the peripapillary ring (p<0.001) and average peripapillary area (p<0.001) decreased as the clinical manifestations of DR worsened (figure 2). VLD in the peripapillary ring, the average peripapillary area and each subquadrant of the ONH were all significantly lower in the DR group than in the non-DR group (all p<0.05), while the VDI of the peripapillary ring was slightly higher in the DR group than the non-DR group (p=0.0289; table 2).
Boxplots showing the distribution of vessel density (VD) in the peripapillary ring (circ) (A) and the entire peripapillary area (average) (B) by status of diabetic retinopathy. DR, diabetic retinopathy; ONH, optic nerve head.
Comparison of peripapillary retinal microcirculation and RNFL between diabetes mellitus patients with or without diabetic retinopathy
In the multivariable linear regression model, the participants with moderate and severe DR showed progressively decreased VD in the peripapillary ring (β=−0.72 for moderate, 95% CI = −1.30 to −0.14, p=0.015; β=−1.79 for severe, 95% CI=−2.81 to −0.77, p=0.001), average peripapillary area (β=−0.74 for moderate, 95% CI=−1.24 to −0.25, p=0.003; β=−1.78 for severe, 95% CI=−2.65 to −0.91, p<0.001), nasal quadrant (β=−1.09 for moderate, 95% CI=−2.11 to −0.07, p=0.037; β=−2.39 for severe, 95% CI=−4.18 to −0.59, p=0.009) and inferior quadrant of the ONH (β=−1.14 for moderate, 95% CI=−2.16 to −0.11, p=0.03; β=−2.00 for severe, 95% CI=−3.81 to −0.20, p=0.03) compared with those without DR. Similar changes were observed between the peripapillary VLD and moderate and severe DR (all p<0.05). Mild DR tended to have increased VLD in the whole image (β=0.55, 95% CI=0.03 to 1.06, p=0.037) and increased VDI (β=0.002, 95% CI=0.00004 to 0.003, p=0.044) (table 3).
Multivariable linear regression of peripapillary retinal microcirculation and RNFL with various stages of DR
Peripapillary retinal nerve fibre layer and diabetic retinopathy
The DR participants had significantly thinner RNFL in the whole peripapillary area (p=0.0053) and inferior quadrant (p=0.0006) than non-DR participants (table 2). In the multivariable linear regression model, moderate (β=−4.56, 95% CI=−8.97 to −0.15, p=0.043) and severe DR (β=−10.12, 95% CI=−18.29 to −1.95, p=0.015) showed progressively thinned peripapillary RNFL in the inferior quadrant, while severe DR appeared to have thicker peripapillary RNFL in the nasal quadrant (β=8.61, 95% CI=2.04 to 15.18, p=0.01). No statistically significant differences were observed with respect to the other RNFL (table 3).
Association between the retinal microvasculature and the retinal nerve fibre layer in the peripapillary region
The RNFL tended to be linearly correlated with VD and VLD, both in the peripapillary ring and average peripapillary area (online supplemental figure 1). The multivariable linear regression analyses demonstrated that both VD and VLD in the peripapillary ring (β=0.60 for VD, 95% CI=0.39 to 0.82, p<0.001; β=0.65 for VLD, 95% CI=0.33 to 0.97, p<0.001) and average peripapillary area (β=1.21 for VD, 95% CI=0.96 to 1.46, p<0.001; β=1.31 for VLD, 95% CI=0.95 to 1.67, p<0.001) had a statistically significant positive correlation with the peripapillary RNFL (table 4). With regard to the subquadrants, VLD positively correlated with the RNFL only in the nasal quadrant (r=0.460, p=0.003; online supplemental table 1).
Supplemental material
Supplemental material
Univariable and multivariable linear regression analyses of average peripapillary RNFL and peripapillary retinal microcirculation
Discussion
The tight neurovascular coupling in the radial peripapillary capillary (RPC) area, which is distributed radially towards the ONH, makes the RPC the focal location of retinal neurodegeneration in the pathophysiology of DR. This study with a large scale of Chinese diabetic patients was performed with a view to better understand the possible relationship of retinal microvascular and neuronal changes with DR. The findings of this study are as follows: (1) compared with the non-DR participants, there was a progressive reduction of VD and VLD in the peripapillary area as DR progressed, (2) the thinner peripapillary RNFL in the inferior quadrant was associated with moderate and severe DR and (3) lower VD and VLD were linearly associated with thinner RNFL in the peripapillary ring and average peripapillary area.
Previous studies on the association between VD and DR severity are limited.14–16 Shin et al found that non-DR and non-proliferative DR exhibited a significantly lower peripapillary VD than healthy eyes without DM.15 Similarly, Lee’s study also indicated a decreased peripapillary VD in DM patients compared with those without DM and significantly lower VD in patients with DM durations of more than 10 years than in those with DM durations of less than 10 years.16 Vujosevic et al demonstrated that a significantly decreased VD occurred earlier in the peripapillary region than in the macular region, even in DM patients without DR,14 suggesting that peripapillary microvascular alterations are important for early DR detection. However, these studies had non-Chinese participants and small sample sizes. In addition, Vujosevic did not adjust for any potential confounders, while Shin made adjustments only for the macular ganglion cell-inner plexiform layer and RNFL. In the present large-scale study, we also found that VD was significantly reduced with the progression of DR after adjusting for systemic and ocular factors.
Similarly to VD, we also identified a progressive reduction in VLD with the progression of DR. VLD is an index of retinal vessel length that removes the disproportionate influence of larger vessels on density calculations to reflect primarily small capillary changes. Both the peripapillary VD and VLD indicated similar decreasing trends with DR progression, indicating a generalised reduction of RPC perfusion in both large vessels and small capillaries. Therefore, monitoring changes in the peripapillary microvasculature is useful for the detection of DR progression. However, peripapillary VDI, an index of vascular calibre, was slightly higher in the DR group in the current study, which is consistent with the results of previous studies that indicated that increased VDI correlated with higher fasting glucose levels.27 28 We hypothesised that a hypoxic glucose environment or increased local inflammatory molecules induces a compensatory vasodilation of the capillaries. By contrast, an observational study with small samples found that VDI is reduced in DR, without adjustments for any confounding factors.29 Moreover, blood vessel calibre measurements on OCTA imaging may not be accurate.30
Diabetic retinal neurodegeneration can occur in the early stages of DR or even before the onset of DR. Our study indicates that the RNFL in the inferior segment yielded a significant loss in the eyes with moderate and more severe DR compared with those without DR. A study by Novara also reported statistically significant differences in the RNFL of the inferior quadrants between DR, non-DR and normal controls.14 Lee et al reported that the DM group had a thinner inferior RNFL than the healthy group, and this change was more severe in patients with DM for longer than 10 years but had no DR.16 The possible mechanism of the higher susceptibility in the inferior sector may be that the inferior peripapillary area is more vulnerable to ischaemic insults because the inferior sector of the ONH had lower blood flow than the superior sector.31 32 Taken together, these results illustrate that the RNFL in the inferior quadrant may be an important biomarker, indicating that RNFL loss in DR eyes may occur early in the inferior sector.
The exact relationship between retinal microvascular and neuronal changes in the presence and progression of DR remains undetermined. A study in Helsinki found no correlations between the peripapillary perfusion parameters (vessel area density and VDI) and the RNFL among 51 diabetic patients.29 In contrast, we identified a significant linear association between the peripapillary retinal microvasculature (VD and VLD) and the RNFL, which is consistent with the findings of several other studies.14–16 33 Lee et al found that peripapillary VD was significantly associated with peripapillary RNFL in 200 individuals with diabetes.16 Moreover, Mase et al demonstrated that the RPC is proportional to the RNFL thickness and plays an important role in nourishing the RNFL.33 The correlation between the microvascular index and the peripapillary RNFL reflects that these two factors may interact with each other during DR progression. Once diabetes damages the retinal microvasculature, subsequent neuronal death and glial dysfunction can impair neurovascular coupling, which in turn causes progressive neurovascular coupling dysfunction and a breakdown of the blood-retinal barrier, reducing retinal metabolic activities and creating a vicious cycle.34 35 Monitoring changes in the peripapillary microvasculature and RNFL using OCTA may be a promising tool in DR screening and treatment. However, the causal effect of neuronal and vascular damage in DR progression could not be determined in the current study owing to its cross-sectional design. Therefore, future longitudinal studies are needed to illustrate the temporal sequence relationship between microvasculature reduction and RNFL loss and to further investigate the possibility of adding neuronal damage to the classification of DR.
The strength of the current study lies in its large sample of treatment-naive DM participants, detailed ocular and systemic examinations with a standardised study protocol and adjustment for several important confounders. However, our study also has several limitations. First, as it is a cross-sectional study, temporality could not be accounted for. It was difficult to illustrate the causal relationship between peripapillary RNFL thinning and decreased peripapillary perfusion. Second, it is a community-based study with participants from a single city. Therefore, generalisations to other areas and ethnicities are limited. Third, severely sick individuals were excluded from the study population. Thus, we believe that the results might have underestimated the association but still have an important reference value. Fourth, the probability of a type I error would increase because multiple statistical tests were performed. Fifth, although great efforts have been made to ensure the accuracy of the OCTA measurements, it is still potentially affected by systemic resolution, image artefacts and motion artefacts which cannot be completely eliminated. 36 37Additionally, slow or stagnant blood flow may not be detected, leading to misestimation of feature analysis.6 Finally, we could not explore the association of the peripapillary retinal layers with DR in a larger region because only 3 mm × 3 mm scanned ONH images were included.
In conclusion, this study found that moderate and severe DR are associated with progressive reductions in the peripapillary retinal microvascular index and RNFL loss in the inferior quadrant. The linear relationship between peripapillary microvascular changes and RNFL loss in various DR stages indicates the coexistence of neuronal and microvascular damage during DR progression. Our study indicates that monitoring the differences in the peripapillary microvasculature and RNFL using OCTA may be a promising approach to detecting DR progression, which should be further investigated in longitudinal studies in other areas and races.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Zhongshan University Ethics Review Board (2017KYPJ094) and was conducted in accordance with the tenets of the Helsinki Declaration. Participants gave informed consent to participate in the study before taking part.
References
Footnotes
JL and DK are joint first authors.
Contributors Study conception and design: LW, MC and WH. Analysis and interpretation: JL, ZX and QX. Writing of the article: JL and DK. Critical revision of the article: JL, LW and WW. Data collection: WW, WH, LW, SC, SZ, JL and XH. Administrative, technical or logistic support: LW and WH. LW is the guarantor.
Funding This research was supported by the National Natural Science Foundation of China (82301236, 82102993), Guangdong Basic and Applied Basic Research Foundation (2021A1515110775, 2023A1515012454), Guangzhou Science and Technology Planning Project (2024A03J0337), the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (303060202400362),the Research and Development Foundation of Dongguan People's Hospital (K201917, K202008) and Guangdong Sci-tech CommissonerCommissioner (20211800500402).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.