Article Text
Abstract
Introduction Severe septic cardiomyopathy (SCM) is one of the main causes of refractory septic shock (RSS), with a high mortality. The application of venoarterial extracorporeal membrane oxygenation (ECMO) to support the impaired cardiac function in patients with septic shock remains controversial. Moreover, no prospective studies have been taken to address whether venoarterial ECMO treatment could improve the outcome of patients with sepsis-induced cardiogenic shock. The objective of this study is to assess whether venoarterial ECMO treatment can improve the 30-day survival rate of patients with sepsis-induced refractory cardiogenic shock.
Methods and analysis ExtraCorporeal Membrane Oxygenation in the therapy for REfractory Septic shock with Cardiac function Under Estimated is a prospective, multicentre, non-randomised, cohort study on the application of ECMO in SCM. At least 64 patients with SCM and RSS will be enrolled in an estimated ratio of 1:1.5. Participants taking venoarterial ECMO during the period of study are referred to as cohort 1, and patients receiving only conventional therapy without ECMO belong to cohort 2. The primary outcome is survival in a 30-day follow-up period. Other end points include survival to intensive care unit (ICU) discharge, hospital survival, 6-month survival, quality of life for long-term survival (EQ-5D score), successful rate of ECMO weaning, long-term survivors’ cardiac function, the number of days alive without continuous renal replacement therapy, mechanical ventilation and vasopressor, ICU and hospital length of stay, the rate of complications potentially related to ECMO treatment.
Ethics and dissemination The trial has been approved by the Clinical Research and Application Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (2020-hs-51). Participants will be screened and enrolled from ICU patients with septic shock by clinicians, with no public advertisement for recruitment. Results will be disseminated in research journals and through conference presentations.
Trial registration number NCT05184296.
- Patients
- Cardiomyopathy
- INFECTIOUS DISEASES
- Adult intensive & critical care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
ExtraCorporeal Membrane Oxygenation in the therapy for REfractory Septic shock with Cardiac function Under Estimated (ECMO-RESCUE) is a prospective, multicentre, non-randomised, cohort study on the application of ECMO in sepsis-induced refractory cardiogenic shock.
Patients admitted to intensive care unit and meet diagnostic criteria for cardiogenic shock induced by sepsis will be enrolled in the study and subsequently assigned to one of two cohorts based on their willingness to undergo ECMO.
All patients willing to undergo ECMO treatment will initiate venoarterial ECMO within 6 hours of enrolment.
Risks of bleeding, thrombosis, leg ischaemia and cannulation-related injuries may be elevated in patients undergoing ECMO treatment; however, this intervention may offer a potential benefit to improve survival.
A limitation of the study is its non-randomised design, which would yield bias.
Introduction
Sepsis, a life-threatening syndrome with organ dysfunction caused by infection, is a leading cause of death in intensive care unit (ICU).1 The global burden of disease arising from sepsis was estimated at 30 million episodes and 6 million deaths per year in 2017.2 The mortality is as high as 50% when septic shock is present.3 4 Septic cardiomyopathy (SCM) is one of the main causes of septic shock, affecting 20%–65% of patients with sepsis. Severe cardiac function impairment leads to refractory septic shock (RSS), with a mortality up to 70%.5–7 However, there are no evidence-based recommendations for the management of SCM.8
In addition to aggressive treatment with antibiotics for infection control, adequate fluid resuscitation and vasoactive drug administration according to the International Guidelines for Management of Sepsis and Septic Shock,9 a variety of drugs to improve cardiac function have also been attempted for the treatment of SCM. However, the results were not satisfactory. Studies on dobutamine have demonstrated that the administration of either dobutamine alone or a combination of norepinephrine failed to improve survival rate, microcirculatory perfusion and metabolic despite an increasing cardiac index, heart rate and left ventricular ejection fractions (LVEF).10 11 Another positive inotropic drug, levosimendan, also has been tried to apply in the treatment of SCM. However, the therapeutic effect is still controversial.12–14 Therefore, there are few recognised effective treatments for sepsis-induced cardiogenic shock at present.
SCM is considered as a sepsis-associated acute syndrome of cardiac dysfunction unrelated to ischaemia.15 Most studies have suggested that recovery from SCM is prompt. A retrospective study showed that although left ventricular dysfunction may persist in approximately one-third of patients with severe sepsis and septic shock in long-term follow-up, the survival rates did not differ.16 Therefore, trying new, innovative therapeutic approaches in SCM are valuable and urgently needed.
Venoarterial extracorporeal membrane oxygenation (ECMO) is a circulatory support technology, which can increase cardiac output as well as improve hypoxemia by increasing oxygen delivery. Therefore, the application of venoarterial ECMO in the treatment of RSS has theoretical feasibility. However, sepsis has been considered as a contraindication for ECMO due to its complexity and the unsatisfactory outcomes of earlier studies. As an extracorporeal circulation device, ECMO may be susceptible to pathogen attachment, leading to refractory infections that exacerbate the underlying condition.17 Furthermore, patients with sepsis often present with thrombocytopenia and abnormal coagulation function, while ECMO necessitates anticoagulation therapy, which can potentially worsen bleeding.18 19 In Park et al’s study, a total of 32 patients received ECMO support for RSS, only seven patients (21.9%) survived to hospital discharge.20 In Huang et al’s study, hospital survival rate was much lower (15%).21
However, as the advancement of ECMO technology, improvements in materials of ECMO22 and the emergence of new research findings, there is a growing reconsideration regarding the utilisation of ECMO in sepsis. It is reported that in neonatal and children with septic shock, the survival rate of ECMO treatment was nearly 80% and 50%, respectively.23 24 ECMO treatment of septic shock in neonatal and children has been in guidelines and consensus since 2008.25 26 Besides this, a single-centre retrospective study found that venoarterial ECMO rescued more than 70% of the patients who developed refractory cardiovascular dysfunction during severe bacterial septic shock.27 Another multicentre cohort study found that survival at 90 days for patients with severe sepsis-induced cardiomyopathy who received venoarterial ECMO was significantly higher than for controls (60% vs 25%).28 Falk et al reported a 90% hospital survival rate in septic shock patients with left ventricular failure and 64.7% in patients with distributive shock.29 Cardiac depression, vasoplegia and capillary leakage all lead to circulatory failure in septic shock. Falk et al’s study suggested that the patients with sepsis-induced refractory cardiogenic shock may receive high survival benefit from venoarterial ECMO and superior to distributed shock. Moreover, all survivors restored their cardiac function and had good long-term quality of life.30 A meta-analysis reported by Ling et al found that survival among patients with LVEF <20% was significantly higher than those with LVEF >35% (62.0% vs 32.1%), and patients with LVEF between 20% and 35% had intermediate survival (42.3%).31 These also implied that perhaps RSS is not an absolute contraindication to ECMO, but rather that the ideal candidates for this treatment should be identified.
In summary, the use of ECMO in adult patients with RSS is still controversial. More studies are still needed to determine whether the benefit of ECMO outweighs the risk. To date, prospective trials of using ECMO in adult patients with sepsis-induced cardiogenic shock have not yet been reported. Therefore, prospective clinical studies would encounter numerous challenges. For example, before ECMO is initiated what dose of vasoactive drug is needed? Are there any other indicators of cardiac function that help to determine the initiation of ECMO in addition to the level of LVEF? This prospective cohort study was designed with reference to indications of ECMO initiation in refractory cardiogenic shock. In this study, we aimed to assess whether venoarterial ECMO treatment can improve the 30-day survival rate of patients with sepsis-induced refractory cardiogenic shock.
Methods and analysis
Study design, setting and patient population
The ExtraCorporeal Membrane Oxygenation in the therapy for REfractory Septic shock with Cardiac function Under Estimated study is a prospective, multicentre, non-randomised, cohort study. All patients admitted to the ICUs of participating centres will be considered as potential candidates for the study. Once the patient is diagnosed of septic shock, he/she should be screened for eligibility by the physicians. When the patient fulfils the criterion of recruitment, the researcher provides details on the purpose, specific content and instructions on how to complete the trial. After we obtain written informed consent (online supplemental file 1) from the patient or a responsible surrogate, patients are enrolled in the study.
Supplemental material
During the period of study, the participants’ legal representative can decide whether to accept ECMO based on their personal conditions. If the participant is confirmed to receive ECMO treatment, we initiate ECMO within 6 hours. Participants taking ECMO during the period of study are referred to as cohort 1, and patients receiving only conventional therapy without ECMO belong to cohort 2. ECMO is established and managed by a professional team. The teams of each centre have more than 5-year experience.
The study will be conducted in six ICUs located in Guangdong, China. It is anticipated that the study will span a duration of 3 years. Participant recruitment commenced in May 2023, with an anticipated completion date for enrolment set for December 2025. The anticipated completion time for followed-up is projected to be in May 2026.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination of our research. The results will be available to the public if necessary.
Inclusion criteria
Age between 18 and 75 years.
Patients admitted into ICU and diagnosed as septic shock (sepsis-3.0), after adequate fluid resuscitation, high-dose vasoactive drug application (vasoactive inotropic score (VIS) >120) and conventional therapy together with at least one of the following criteria: (1) sustained hypotension (mean arterial pressure (MAP) <65 mm Hg); (2) persistent lactacemia (two consecutive values >5 mmol/L with at least 30 min interval between samples), with non-decreasing trend on steady doses of inotropes and/or vasopressors; (3) persistent low central venous blood oxygen saturation (ScvO2) (two consecutive values <55% with at least 30 min interval between samples), with non-increasing trend on steady doses of inotropes and/or vasopressors. The above condition lasts more than 5 hours.
Rapidly deteriorating sepsis-induced myocardial impairment is defined by at least one of the following criteria: (1) rapidly deteriorating ventricular function (LVEF <35%); (2) CI <2.0 L/min/m2 (>3 hours); (3) emerging refractory arrhythmia.
Informed consent provided by the patient or person with decisional responsibility.
Exclusion criteria
Cardiac dysfunction caused by other causes is excluded, such as acute myocardial infarction, chronic heart failure, congenital cardiac disease, myocardial effusion, moderate to severe aortic regurgitation, severe aortic coarctation and so on.
High suspicion of pulmonary embolism, tension pneumothorax or cardiac tamponade as a cause of shock.
Prolonged cardiac arrest (>30 min) before ECMO, or cardiopulmonary resuscitation survivors remaining comatose.
Irreversible condition or meet the inclusion criteria for more than 12 hours.
Presence of active bleeding or anticoagulant contraindications.
Peripheral artery disease disabling insertion of outflow cannula to femoral artery.
Irreversible neurological pathology.
Severe underlying condition with lift expectancy less than 1 year.
Special population, such as pregnancy, AIDS.
Patient included in another interventional clinical trial.
Study definitions
Septic shock
Septic shock, a subset of sepsis, can be identified by a vasopressor requirement to maintain an MAP of 65 mm Hg or greater and serum lactate greater than 2 mmol/L despite of resuscitation.
Vasoactive inotropic score
VIS was calculated as ((epinephrine+norepinephrine) μg/kg/min)×100+((dobutamine+dopamine) μg/kg/min)+(milrinone μg/kg/min)×10+levosimendan μg/kg/min×50+(vasopressin units/kg/min)×10 000.32
Successful weaning off ECMO
Successful weaning was defined as maintaining stable condition within 24 hours of ECMO weaning.
Study intervention
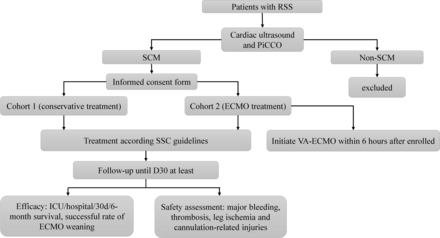
The study flowchart is detailed in figure 1.
Study flowchart. ECMO, extracorporeal membrane oxygenation; PiCCO, pulse indicator continuous cardiac output techonology; RSS, refractory septic shock; SCM, septic cardiomyopathy.
All patients received fluid resuscitation, antibiotic, vasoactive drugs and control of the focus of infection according to the international guidelines of Surviving Sepsis Campaign 2021.9 The gold of MAP is ≥65 mm Hg. For multiple-organ dysfunction, life support technologies such as mechanical ventilation and continuous renal replacement therapy (CRRT) are provided as needed. The patient’s primary physicians will determine the management of other comorbidities.
ECMO implantation and management
All patients in cohort 1 will initiate ECMO as fast as possible. A maximum of 6 hours is allowed between enrolment and the actual initiation of ECMO. ECMO catheterisation and management will be operated by an experienced ECMO team and carried out at the bedside. The initiation and weaning or cessation time, the data of ECMO will be recorded by nurses.
Therapy mode
Venoarterial or venovenoarterial mode will be chosen according to the patient’s condition. Intra-aortic balloon pump will be performed simultaneously when necessary to relieve the afterload of left ventricle.
Canulation
All patients will undergo peripheral cannulation. The arterial catheter is placed into the femoral artery, and the venous catheter is placed into the femoral vein. Ultrasound examination will be performed bedside to select vessels with better conditions and suitable diameter canula before catheterisation. After the arterial cannulation, the distal perfusion catheter is inserted to perform lower limb perfusion.
The blood flow and the goal
The initial flow rate is 80~100 mL/kg of ideal body weight/min. The ECMO blood flow is adjusted to: (1) maintain a MAP >65 mm Hg; (2) reach a preoxygenator oxygen saturation >65%; (3) restore blood lactate to normal level; (4) revert the multiple organ dysfunction syndrome (MODS).
Management
Inotropes are discontinued or reduced to minimal dosed within a few hours of achieving goal-directed flows (norepinephrine or epinephrine <0.05 µg/kg/min, and dopamine or dobutamine <5 µg/kg/min are suggested).
During the period of ECMO, maintain a haemoglobin of >70 g/L, a platelet count of ≥50×109 /L, antithrombin III (AT3) of >80% and fibrinogen >2 g/L.
Echocardiography is performed at least daily to monitor cardiac function.
Anticoagulation
Unfractionated heparin (UFH) is recommended for anticoagulation. A bolus dose of UFH is administered at cannulation followed by continuous infusion. Activated partial thrombin time is targeted between 1.5 and 2 times of normal, or active clotting time (ACT) is targeted between 180 s and 220 s. If the patient is at high risk of bleeding, the target ACT value is lowered to 160 s. UFH may be stopped for severe bleeding or coagulation disorders.
Wean off ECMO
ECMO weaning should be considered when patients exhibit stable haemodynamics and sufficient cardiac recovery.33
Indications for ECMO weaning: (1) adequate upper limb partial pressure of oxygen in arterial blood and saturation with fraction of inspire oxygen <50%, peak inspiratory pressure <30 cm H2O, positive end expiratory pressure <8 cmH2O from ventilator when ECMO gas flow at 21%; (2) ECMO flow is reduced to 10%~25% of normal blood flow or 1.5 L/min; (3) patients exhibit stable haemodynamic (MAP >65 mm Hg, pulse pressure >20 mm Hg), ScvO2 >70%, LVEF >40%, blood lactate <2.0 mmol/L and without malignant arrhythmia on no/low doses vasoactive, inotropic support (norepinephrine or epinephrine <0.02 µg/kg/min, and dopamine or dobutamine <5 µg/kg/min) for more than 2 hours.
The ECMO weaning test is gradually performed according to the patient’s systemic haemodynamics and tissue perfusion improvement.33 34 During weaning, ECMO flow is decreased progressively by 500 mL every 5–10 min. Patients are evaluated after 3–5 min of no support (circuit clamped) or alternatively at minimum of 1 L/min of support. Successful weaning is defined as maintaining stable condition within 48 hours of ECMO weaning.
Cessation of ECMO
ECMO will be discontinued with one of the following conditions: (1) brain death; (2) other vital organ dysfunction is difficult to reverse; (3) major bleeding (defined as fatal bleeding, and/or symptomatic bleeding, does ≥1 of the following factors apply: (1) bleeding at a critical site, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial or intramuscular with compartment syndrome; (2) haemodynamic instability; (3) clinically overt bleeding with haemoglobin decrease ≥2 g/dL or administration of ≥2 units red blood cells;35 (4) there are no signs of cardiac function recovery and no better therapeutic regimen after 7 to 10 days of ECMO treatment; (5) an uncontrollable infection.
Primary outcome
The primary outcome is 30-day survival measured from the date of enrolled (D0) until death or day 30. For patients who were discharged alive from ICU, information on the primary endpoint will be acquired by a telephone call.
Secondary outcomes
The secondary outcomes include: (1) survival to ICU discharge, hospital survival, 6-month survival and quality of life for long-term survival (EQ-5D score); (2) successful rate of ECMO weaning; (3) long-term survivors’ cardiac function was evaluated according to Doppler echocardiography; (4) the number of days alive without CRRT, mechanical ventilation and vasopressor (the numbers of CRRT-free days, mechanical ventilation-free days and vasopressor-free days, between D0 and up to D30); (5) ICU and hospital length of stay.
Safety assessments
In addition to the focus on prognosis of patients with SCM treated with venoarterial ECMO, the safety of the venoarterial ECMO treatment is also a major focus. The complications potentially related to ECMO treatment include: (1) major bleeding associated with anticoagulants; (2) thrombosis (ischaemic stroke, pulmonary embolism, deep venous thrombosis or catheter-associated thrombosis during study confirmed by ultrasound or CT scan); (3) leg ischaemia; (4) cannulation-related injuries (such as arterial laceration, arterial aneurysm and peripheral nerve defect).
Sample size
According to a retrospective study from Bréchot et al, the survival rate of patients with severe sepsis-induced cardiomyopathy was 60% in venoarterial ECMO group and 25% in control group, elevation in survival of 35% in venoarterial ECMO group can be expected. Considering the high cost and uncertain therapeutic effect of ECMO treatment, fewer participants may choose ECMO treatment than conventional treatment, with an estimated ratio of 1:1.5. The sample size for differences between two independent proportions was calculated by the PASS V.14.0 software to ensure 80% power using a two-sided test with a significance level of α=0.05. We need 23 participants in venoarterial ECMO treatment cohort and 35 participants in conventional treatment cohort. Considering a projected dropout rate of 10%, the sample size of venoarterial ECMO treatment cohort and conventional treatment cohort should be 25 and 39, respectively. The total sample size should be 64.
Data collection and follow-up
Each investigator from the six participating ICUs was trained to the protocol and data collection in the Case Record Form before trial initiation. The data is managed and closed by the Clinical Research centre of the Second Affiliated of Guangzhou Medical University (China).
Flowchart of patient follow-up is shown in table 1. Demographic data and medical history will be collected. Detailed data including reasons for ICU admission, cause of septic shock, focus of infection, acute physiology and chronic health evaluation II (APACHEII) score, dates of hospital and ICU admission will be recorded. Details of mechanical ventilation, vasopressor and CRRT will be documented daily. Sequential organ failure assessment score (SOFA) will be calculated at baseline, day 1 (D1), D3 and D7. Cardiac function will be assessed by echocardiography and recorded at baseline, D1, D3, D5, D7, D10, D14, D30. Blood will be collected at baseline, D1, D3, D5, D7. The following laboratory results will be recorded: white blood cell count and differentials in peripheral circulation, serum electrolyte levels, liver and myocardial enzyme concentrations, arterial blood gas analysis, C reactive protein, procalcitonin and lactate. EQ-5D assessment will be acquired through phone call at 6 months.
Flowchart of patient follow-up
During ECMO intervention, details of initiation, mode, setting parameters, weaning or cessation, complications will be noted.
All enrolled participants will be followed to determine adverse events, cardiac function recovery and mortality until death or at ICU/hospital discharge, 30 days and 6 months. If the participants survive beyond 30 days and 6 months, they will be requested to revisit the hospital for a cardiac ultrasound examination or provide a cardiac ultrasound report from a local hospital.
Statistical analysis
Data will be double-checked by the clinical research team, and the database is managed and closed by the Clinical Research centre of the Second Affiliated of Guangzhou Medical University (China).
For each cohort, quantitative variables with normal distribution will be described as mean and SD. Quantitative variables with skewed distribution will be described as median (M) and (IQR, 25th percentile to 75th percentile). Qualitative variables will be described as frequency and percentage.
The effect of venoarterial ECMO treatment versus conventional treatment on 30 days survival will be performed using Fisher’s exact test, with secondary analysis by Kaplan-Meier survival analyses, comparison using a log rank test. A propensity score-weighted analysis was done for treatment-effect estimation. Covariate balance between the two groups was assessed after weighting, and we considered an absolute standardised difference of less than 0.1 as evidence of balance. The effect of ECMO on survival at 30 days will be estimated within the weighted pseudopopulation. Adjusted Kaplan-Meier estimator and log-rank test (considering the weighting scheme) will be used. Decision tree analysis will be used to establish a decision tree model to decide whether sepsis-induced cardiogenic shock patients need ECMO treatment.
Safety will be analysed by the frequency of complications in both cohorts and comparing rates using χ2 or Fisher’s exact test, with an alpha risk set at 0.05.
Statistical analyses of the prespecified secondary endpoints will be performed with descriptive and inductive statistical methods. Categorical variables will be compared using the χ2 or Fisher’s exact test, as appropriate. Continuous variables will be compared using Student’s t test or the Mann-Whitney test, as appropriate.
Therapeutic efficiency will be analysed using the data of the full analysis set and per protocol set; safety evaluation will be based on the data of the safety analysis set for statistical analysis.
All analyses were performed with commercially available statistical software SPSS 22.0 and R software version 4.1.0 or later.
Ethics and dissemination
The study protocol has been approved by the Clinical Research and Application Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (V.2.0, registration number: 2020-hs-51; date of approval: 17 November 2020). Participants will be screened and enrolled from ICU patients with sepsis-induced cardiogenic shock by clinicians, with no public advertisement for recruitment. When the patient fulfils the criterion of recruitment, written informed consent (online supplemental file 1) should be obtained from the patient or a responsible surrogate before enrolled. After enrolled, the participants can decide whether to accept ECMO based on their personal conditions. All information from the participants will be kept private and will not be provided to any company or institution. Results will be disseminated in research journals and through conference presentations.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
W-yC and Z-bG contributed equally.
D-lW and Z-hZ contributed equally.
Contributors W-yC and Z-bG designed the trial. T-yK, QY, Y-cW and X-mX participated in the discussion of the plan, D-lW and Z-hZ made a decision to revise the plan. Z-bG, T-yK, W-xC, X-hC, QY, Y-cW, Q-rW and FZ conduct the plan. W-yC draft the manuscript. D-lW and Z-hZ provided critical revision of the manuscript. D-lW and W-yC obtained funding for the trial. All authors discussed and helped to improve the protocol and read and approved the final manuscript.
Funding This study was funded by a Grant from the Second Affiliated Hospital of Guangzhou Medical University (2020-LCYJ-DZX-02) and Runze Medical Research Fund of Wu Jieping Medical Foundation (320.6750.2023-02-6).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.